chaitea
Rough_Rock
- Joined
- Nov 16, 2022
- Messages
- 13
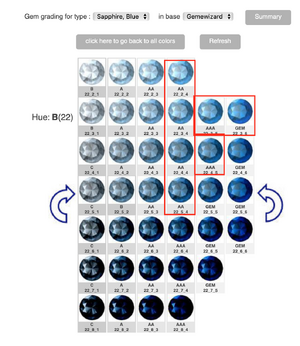
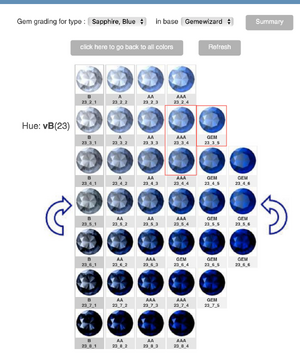
As my title says, does cornflower blue have purple or violet undertones? I ask because I keep reading that pure blue without ANY violet is desirable for sapphires or that cornflowers are the only flowers that are pure blue without undertones. But quite honestly, in my garden, all my cornflowers are a rather deep blue with an unmistakable violet undertone.
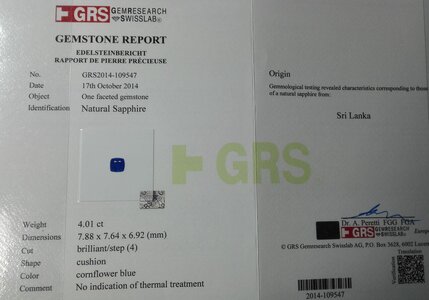
I've also just read that for antique Kashmir sapphires, that a tiny tinge of violet is actually common in Kashmir sapphires. It's by Enhoerning, who specialise in coloured stones (see here https://www.enhoerning-jewelry.com/blog/kashmir-sapphire-faq
Not that I'll purchase a Kashmir sapphire, but I think the colours are lovely in their own way. Is this some sort of attempt to standardise sapphires for pricing differences?
I've also just read that for antique Kashmir sapphires, that a tiny tinge of violet is actually common in Kashmir sapphires. It's by Enhoerning, who specialise in coloured stones (see here https://www.enhoerning-jewelry.com/blog/kashmir-sapphire-faq
Not that I'll purchase a Kashmir sapphire, but I think the colours are lovely in their own way. Is this some sort of attempt to standardise sapphires for pricing differences?





















300x240.png)