Starstruck8
Brilliant_Rock
- Joined
- May 13, 2021
- Messages
- 603
... and all my other questions about opals.
(@yssie, I've started this new thread to avoid derailing the other two.)
It's a fascinating but frustrating article. The key worry is right at the beginning, p34: 'Despite there being no consensus on genesis at the moment...'. As best I can tell, there still is no consensus. I'd love to be proven wrong!
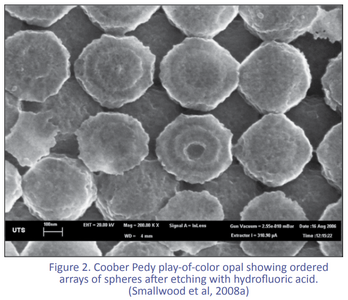
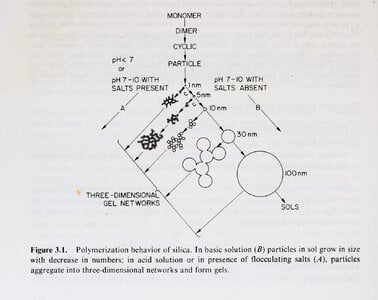
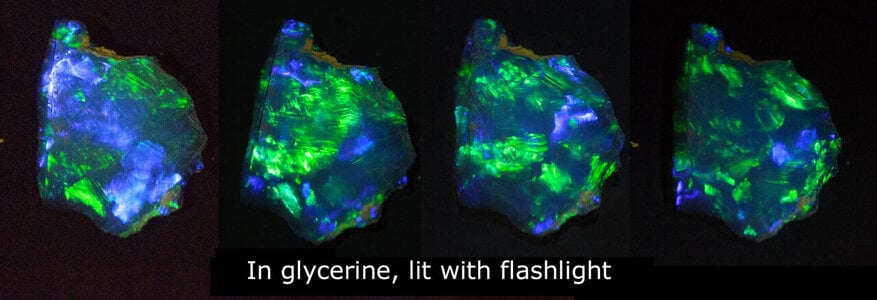
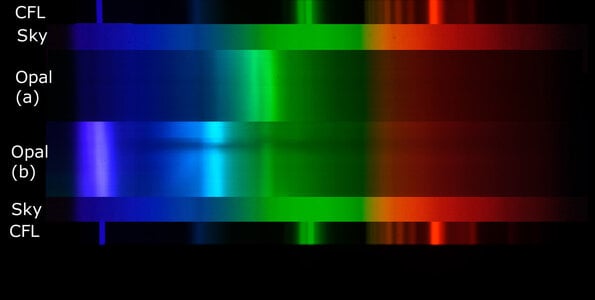
The play of colour in precious opal is caused by silica spheres of roughly uniform size, arranged in regular arrays. But how were the spheres formed? How did they get to be so uniform in size? How were they arranged into neat arrays?
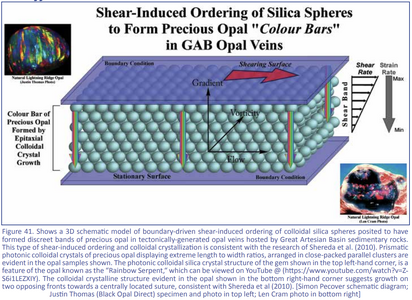
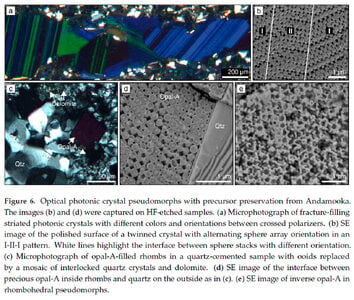
I'll leave aside the question of how the spheres were formed. Pecover is advocating for his theory that the sorting and arranging was done by flow under pressure - fluid containing spheres was forced into cavities under pressure. Irregularities in the flow caused the spheres to start piling up in regular arrays. He is arguing against the 'traditional' view - fluid containing spheres seeped slowly into cavities and the spheres settled slowly to the bottom, building regular arrays from the bottom up. There are also other stories...
In favour of slow-but-steady bottom-up, you certainly can form opal that way - it's how synthetic opal is made:
https://www.synthetic-opals.com/synthetic-opal-manufacture.html
In favour of flow-under-pressure, some opals show obvious flow structures. Pecover gives examples. This article, cited by Pecover, gives others:
https://www.mdpi.com/2075-163X/8/1/12/htm
(Check out the SEM pictures - 'regular' comes in degrees!) But what about opals that don't show obvious flow structures?
So who is right? Or both or neither? I haven't been able to find any authoritative-looking source that suggests a consensus.
All this relates only to Australian (sedimentary) opals. The mostly 'volcanic' opals found in other parts of the world are a whole different story...
I'd love to hear from anyone with interesting questions or information about opals.
(@yssie, I've started this new thread to avoid derailing the other two.)
@Starstruck8 Piggybacking on a convo in another thread - I read this first website and felt like it generated more questions than it answered -
http://opalsociety.org/opal_cutting.htm
More googling, more questionable results... But all reputable pages seem to reference this article - which I've just started to read but seems like it will (actually) answer a lot of the questions we've been tossing out. Interested in your thoughts if you have time to read it! Starts on Pg 34 - Frozen Opal Fluids and Colloidal Crystal Fire: Gem Opal Deposits in the Heart of Australia
https://www.opal.asn.au/wp-content/uploads/2019/01/InColor-41-Winter-2019-complete-22142.pdf
It's a fascinating but frustrating article. The key worry is right at the beginning, p34: 'Despite there being no consensus on genesis at the moment...'. As best I can tell, there still is no consensus. I'd love to be proven wrong!
The play of colour in precious opal is caused by silica spheres of roughly uniform size, arranged in regular arrays. But how were the spheres formed? How did they get to be so uniform in size? How were they arranged into neat arrays?
I'll leave aside the question of how the spheres were formed. Pecover is advocating for his theory that the sorting and arranging was done by flow under pressure - fluid containing spheres was forced into cavities under pressure. Irregularities in the flow caused the spheres to start piling up in regular arrays. He is arguing against the 'traditional' view - fluid containing spheres seeped slowly into cavities and the spheres settled slowly to the bottom, building regular arrays from the bottom up. There are also other stories...
In favour of slow-but-steady bottom-up, you certainly can form opal that way - it's how synthetic opal is made:
https://www.synthetic-opals.com/synthetic-opal-manufacture.html
In favour of flow-under-pressure, some opals show obvious flow structures. Pecover gives examples. This article, cited by Pecover, gives others:
https://www.mdpi.com/2075-163X/8/1/12/htm
(Check out the SEM pictures - 'regular' comes in degrees!) But what about opals that don't show obvious flow structures?
So who is right? Or both or neither? I haven't been able to find any authoritative-looking source that suggests a consensus.
All this relates only to Australian (sedimentary) opals. The mostly 'volcanic' opals found in other parts of the world are a whole different story...
I'd love to hear from anyone with interesting questions or information about opals.




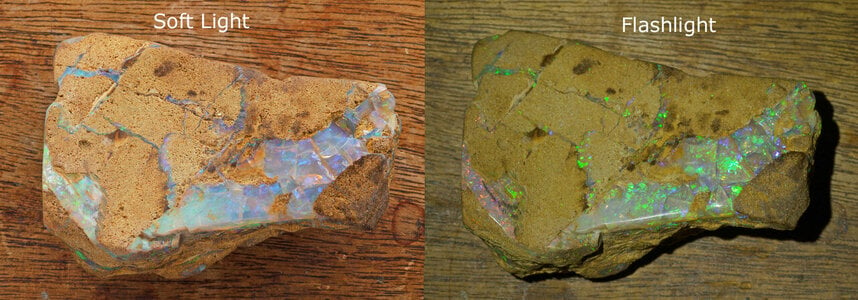


300x240.png)