- Joined
- Apr 23, 2020
- Messages
- 29
Hi everyone,
I have a question as I tried to search up the forum but I haven’t find anything about it.
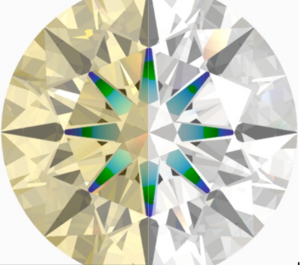
Do you guys have any pictures, videos or links showing the difference between a diamond showing lots of spectra colour and a diamond showing fire?
In particular I’m interested in those diamonds showing lots of rainbow colours when in indirect sunlight.
Does it happen more often when the diamond is colourless vs it has more yellow?
Does it just depend on the cut or is there a specific diamond quality that influences it?
thanks a lot in advance!

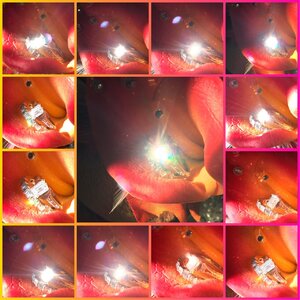
D colour, super ideal cut diamond in indirect sunlight.
I have a question as I tried to search up the forum but I haven’t find anything about it.
Do you guys have any pictures, videos or links showing the difference between a diamond showing lots of spectra colour and a diamond showing fire?
In particular I’m interested in those diamonds showing lots of rainbow colours when in indirect sunlight.
Does it happen more often when the diamond is colourless vs it has more yellow?
Does it just depend on the cut or is there a specific diamond quality that influences it?
thanks a lot in advance!

D colour, super ideal cut diamond in indirect sunlight.





300x240.png)