- Joined
- May 11, 2013
- Messages
- 7,570

US FDA announces emergency authorization for convalescent plasma to treat Covid-19
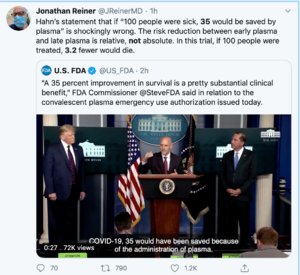
The US Food and Drug Administration on Sunday issued an emergency use authorization for convalescent plasma to treat Covid-19, saying the "known and potential benefits of the product outweigh the known and potential risks of the product."
The US Food and Drug Administration issued an emergency use authorization for the use of convalescent plasma to treat Covid-19 on Sunday, saying the "known and potential benefits of the product outweigh the known and potential risks of the product."
The FDA said more than 70,000 patients had been treated convalescent plasma, made using the blood of people who have recovered from coronavirus infections.
"Today I am pleased to make a truly historic announcement in our battle against the China virus that will save countless lives," President Trump said at a White House briefing. "Today's action will dramatically increase access to this treatment."












300x240.png)