- Joined
- Jul 27, 2009
- Messages
- 4,137
Re: Article: Over Grading of Blue Fluorescent Diamonds Revis
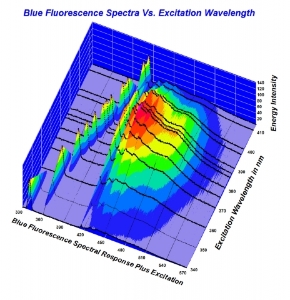
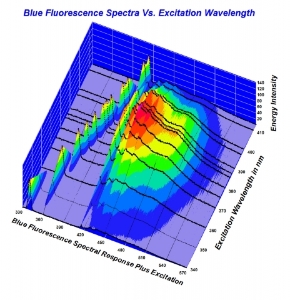
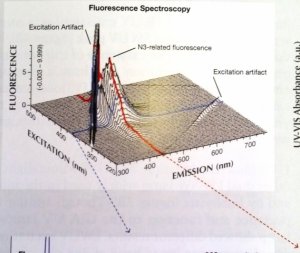
Here is the chart as a screen shot. Higher resolution would be much easier to look at. Also, here is a quick link to the full 2010 study for convenience. (It is linked in the article being disussed here). http://www.acagemlab.com/temp/CowingOvergrading.pdf
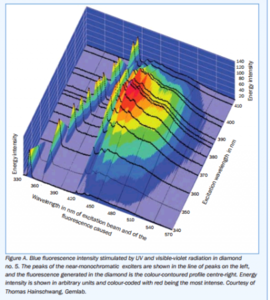
Texas Leaguer|1454592037|3986748 said:Garry H (Cut Nut)|1454555632|3986616 said:Look at this chart - I chopped off an experimental error - You can see there is excitation taking effect right down to very slightly greenish blue.
The 3D graph by Thomas Hainschwang on page 42 of the Cowing study illustrates the same concept, BUT with the added element of "intensity". Those results support the conclusion (same page) "At normal viewing distances from artificial illumination the violet light intensity, just like the UV, is too weak to excite noticeable fluorescence".
I could not manage to copy the chart seperately. Perhaps Michael can post it here.
Here is the chart as a screen shot. Higher resolution would be much easier to look at. Also, here is a quick link to the full 2010 study for convenience. (It is linked in the article being disussed here). http://www.acagemlab.com/temp/CowingOvergrading.pdf





300x240.png)