- Joined
- Jun 8, 2008
- Messages
- 56,364
Happy 2023 all!
"
LONDON (Reuters) - Pandemic restrictions that hampered the circulation of viruses other than COVID-19 could be behind the unseasonably early upsurge in respiratory infections in Europe this winter that the festive break could prolong, scientists say.
Apart from COVID-19, regulations to curb movement and social interaction limited the transmission of viruses that typically cause most infections during the colder, winter months, including influenza and RSV (respiratory syncytial virus).
That created a bigger pool of susceptible people, including children born during this time, who had less exposure to these viruses.
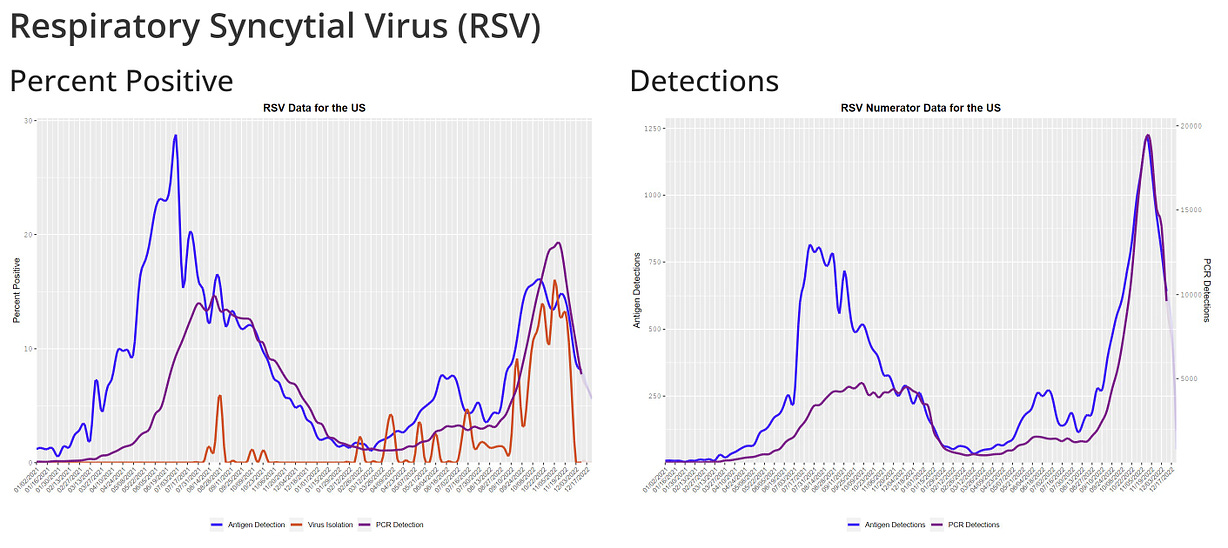
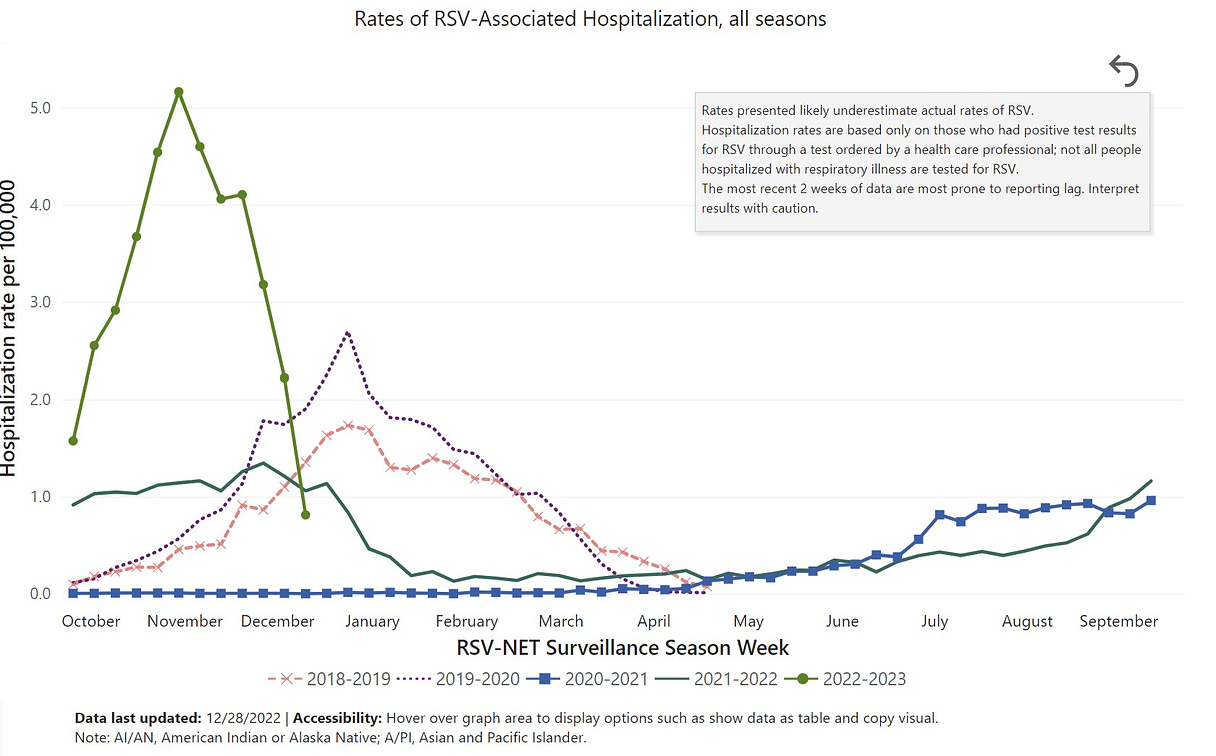
RSV usually causes mild, cold-like symptoms, but can result in serious illness in older adults and young infants.
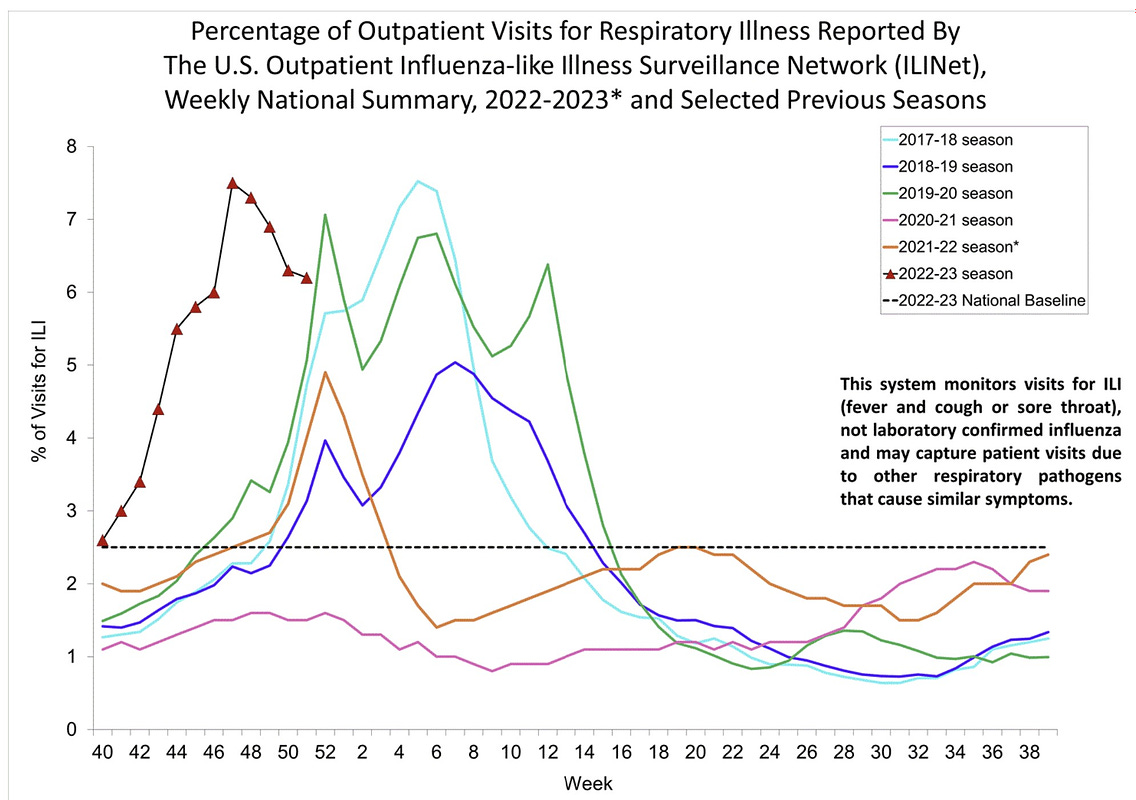
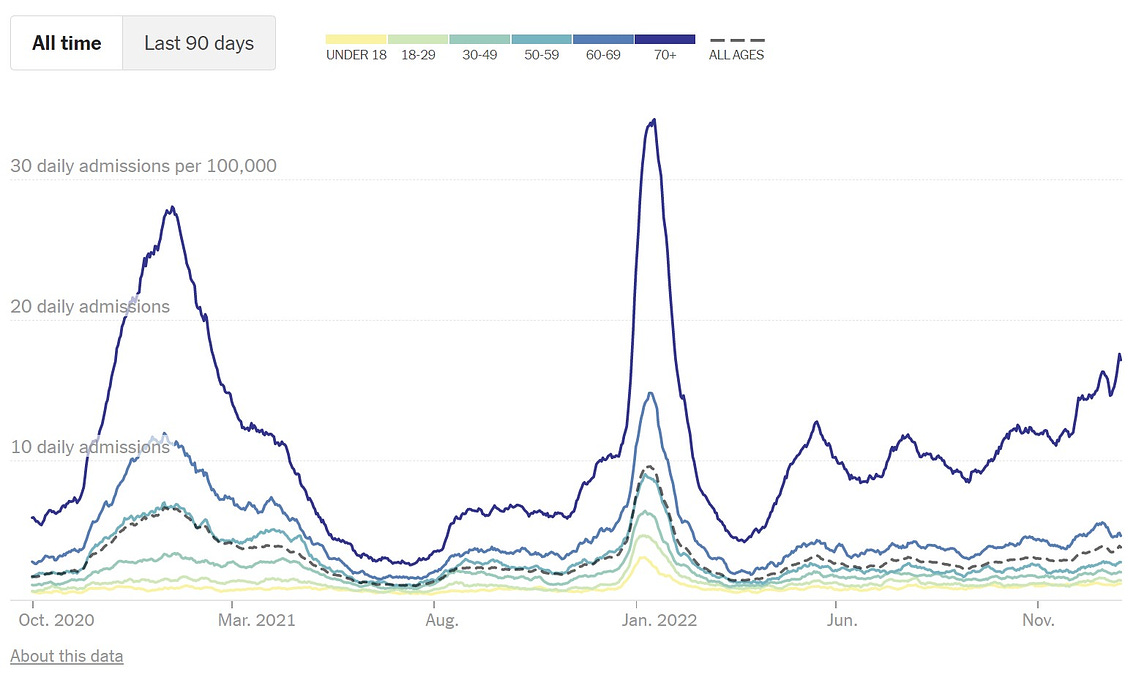
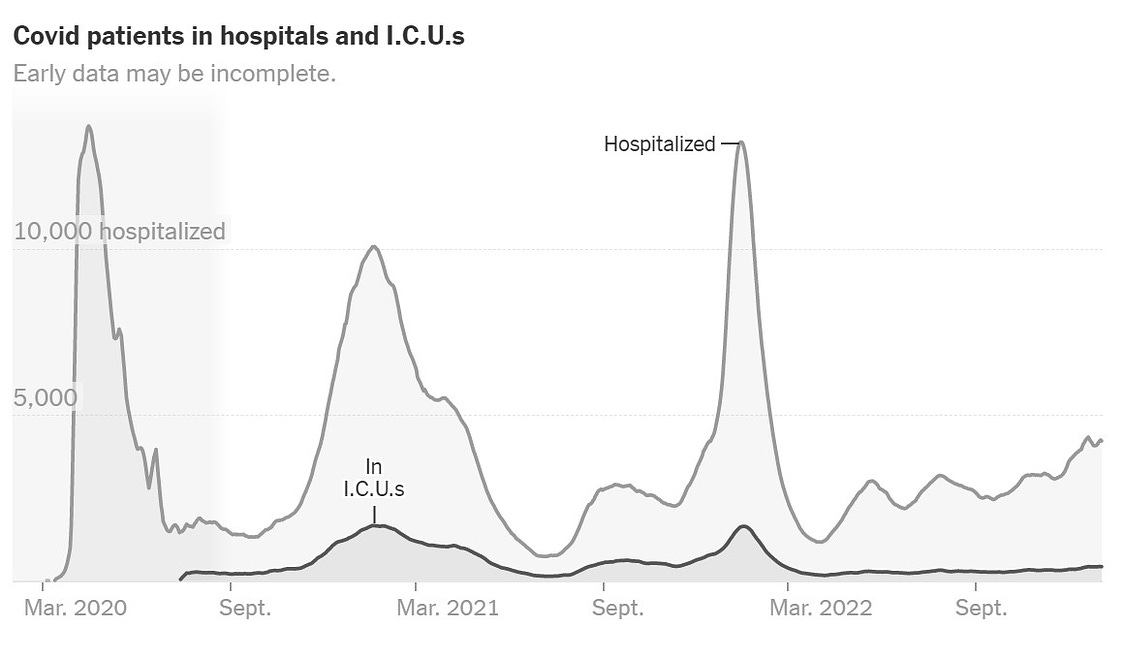
This winter, health officials have warned of what has been dubbed a tripledemic of influenza, RSV and continued COVID-19 cases, adding to the pressure on over-burdened health services.
RSV surveillance data from 15 European countries spanning the pre-COVID years 2010-2011 to 2015-2016 show the median start to the RSV season is early December, and that it peaks around the end of January, the ECDC (European Centre for Disease Prevention and Control) highlighted in a report published this month.
European trends so far suggest that this year RSV cases peaked in late November and are in decline, but there will still be a substantial number of cases in the next four to six weeks, said Agoritsa Baka, the ECDC's expert in emergency preparedness and response.
In Wales, for instance, there were 111.6 confirmed RSV cases per 100,000 in children aged under five in the week ending Nov. 27.
For the 2018-2019 and 2019-2020 season, confirmed cases under the same parameters were below 50. In both those years, even the eventual peaks, which came a few weeks later, were just short of 50.
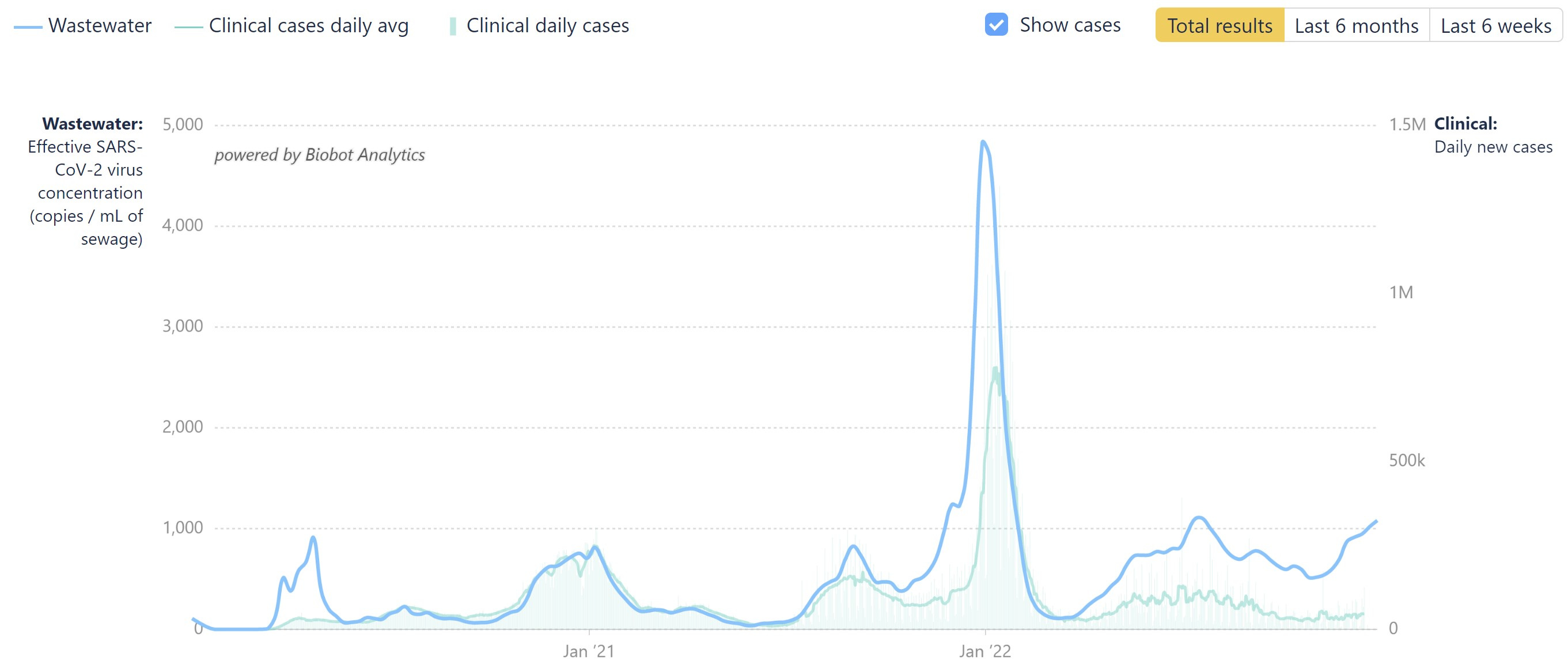
Meanwhile, COVID cases have risen in recent weeks. In the week ended Dec 18, European cases rose 7% over the week prior, according to ECDC figures.
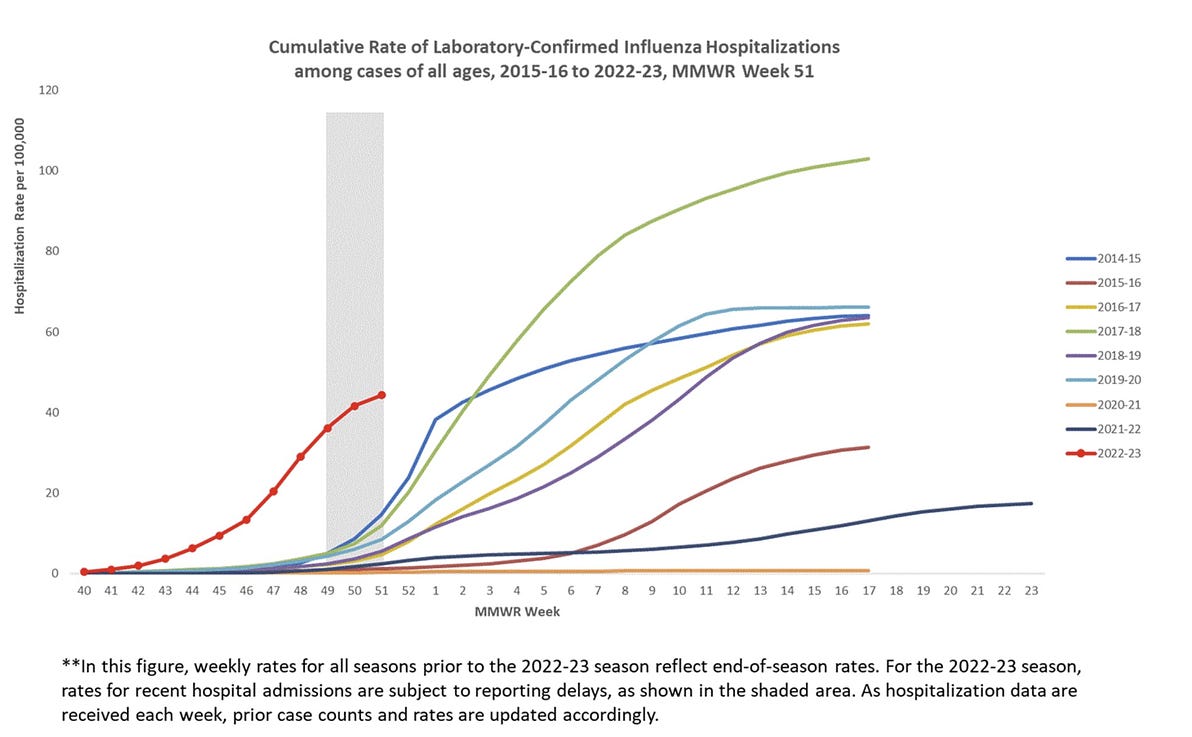
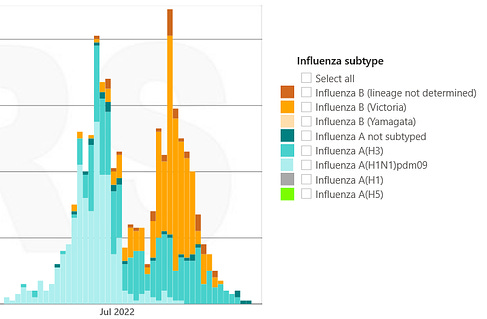
The flu upsurge began in the second week of November in the European region, an earlier start than the four previous seasons, the agency said.
"The accumulation of more susceptible persons in the last two years, plus the increased mixing of people during the summer months (following the easing of restrictions) have contributed to an earlier start of the outbreaks in the current season 2022-2023," Baka said.
"We do not have any direct reference for this statement," she said, but cited a study published by the U.S. Centers for Disease Control and Prevention that linked sharp declines in influenza circulation in the 2020-2021 season to COVID-19 restrictions in the northern and southern hemispheres.
Peter Openshaw, a respiratory physician and professor at Imperial College London, said it was plausible that limited social mixing in recent years had contributed to a "downward drift" in specific immunity to these viruses at a population level, alongside a decline in general immunological responsiveness in people.
UNKNOWN TERRITORY
With little in the way of direct comparison with the situation this year, it is unclear whether sharp peaks earlier than usual will necessarily lead to a higher total number of cases over the season, compared to pre-pandemic years.
But scientists are concerned that social interaction during the festive season could lead to further increases in respiratory infections, especially as people meet vulnerable elderly relatives.
"If you're sick - don't go to a party. Get tested before you go visit your grandmother. And it would be prudent to wear a mask in crowded places particularly in public transport," ECDC’s Baka said.
As an added complication, viral respiratory infections can predispose patients to bacterial infections, just when some common antibiotics that can treat them are in short supply in Europe.
This has been linked to increased demand given an uptick in severe infections caused by a bacteria called group A Streptococcus, particularly in children under the age of ten.
Supply hiccups attributed to long-standing pricing pressure on the manufacture of generic medicines in the continent that has worsened with the energy crisis have added to the shortage.
(Reporting by Natalie Grover in London; Editing by Matt Scuffham and Barbara Lewis)
"
Pandemic Curbs Linked to Early Start to Europe's Winter Flu Season
By Natalie Grover"
LONDON (Reuters) - Pandemic restrictions that hampered the circulation of viruses other than COVID-19 could be behind the unseasonably early upsurge in respiratory infections in Europe this winter that the festive break could prolong, scientists say.
Apart from COVID-19, regulations to curb movement and social interaction limited the transmission of viruses that typically cause most infections during the colder, winter months, including influenza and RSV (respiratory syncytial virus).
That created a bigger pool of susceptible people, including children born during this time, who had less exposure to these viruses.
RSV usually causes mild, cold-like symptoms, but can result in serious illness in older adults and young infants.
This winter, health officials have warned of what has been dubbed a tripledemic of influenza, RSV and continued COVID-19 cases, adding to the pressure on over-burdened health services.
RSV surveillance data from 15 European countries spanning the pre-COVID years 2010-2011 to 2015-2016 show the median start to the RSV season is early December, and that it peaks around the end of January, the ECDC (European Centre for Disease Prevention and Control) highlighted in a report published this month.
European trends so far suggest that this year RSV cases peaked in late November and are in decline, but there will still be a substantial number of cases in the next four to six weeks, said Agoritsa Baka, the ECDC's expert in emergency preparedness and response.
In Wales, for instance, there were 111.6 confirmed RSV cases per 100,000 in children aged under five in the week ending Nov. 27.
For the 2018-2019 and 2019-2020 season, confirmed cases under the same parameters were below 50. In both those years, even the eventual peaks, which came a few weeks later, were just short of 50.
Meanwhile, COVID cases have risen in recent weeks. In the week ended Dec 18, European cases rose 7% over the week prior, according to ECDC figures.
The flu upsurge began in the second week of November in the European region, an earlier start than the four previous seasons, the agency said.
"The accumulation of more susceptible persons in the last two years, plus the increased mixing of people during the summer months (following the easing of restrictions) have contributed to an earlier start of the outbreaks in the current season 2022-2023," Baka said.
"We do not have any direct reference for this statement," she said, but cited a study published by the U.S. Centers for Disease Control and Prevention that linked sharp declines in influenza circulation in the 2020-2021 season to COVID-19 restrictions in the northern and southern hemispheres.
Peter Openshaw, a respiratory physician and professor at Imperial College London, said it was plausible that limited social mixing in recent years had contributed to a "downward drift" in specific immunity to these viruses at a population level, alongside a decline in general immunological responsiveness in people.
UNKNOWN TERRITORY
With little in the way of direct comparison with the situation this year, it is unclear whether sharp peaks earlier than usual will necessarily lead to a higher total number of cases over the season, compared to pre-pandemic years.
But scientists are concerned that social interaction during the festive season could lead to further increases in respiratory infections, especially as people meet vulnerable elderly relatives.
"If you're sick - don't go to a party. Get tested before you go visit your grandmother. And it would be prudent to wear a mask in crowded places particularly in public transport," ECDC’s Baka said.
As an added complication, viral respiratory infections can predispose patients to bacterial infections, just when some common antibiotics that can treat them are in short supply in Europe.
This has been linked to increased demand given an uptick in severe infections caused by a bacteria called group A Streptococcus, particularly in children under the age of ten.
Supply hiccups attributed to long-standing pricing pressure on the manufacture of generic medicines in the continent that has worsened with the energy crisis have added to the shortage.
(Reporting by Natalie Grover in London; Editing by Matt Scuffham and Barbara Lewis)
"

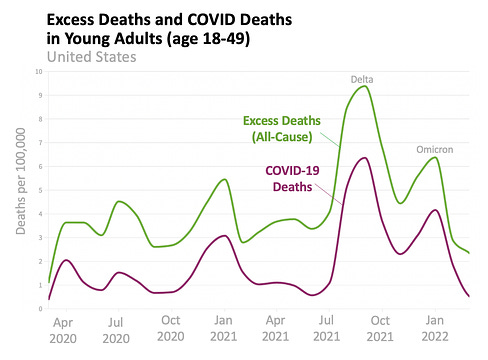
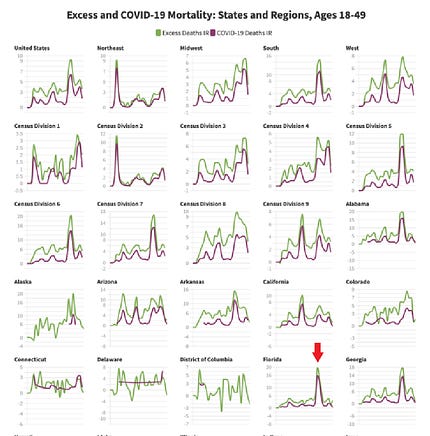
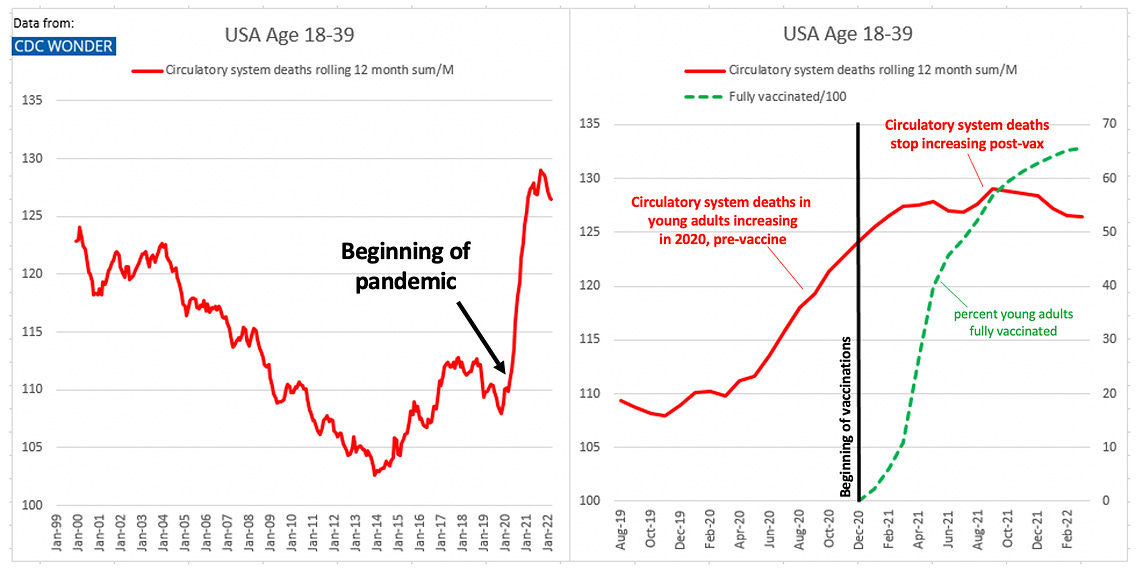
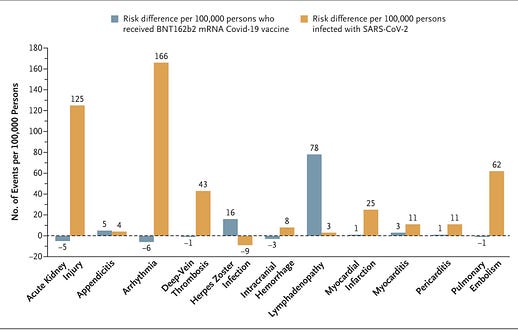


![author['full_name'] author['full_name']](https://clf1.medpagetoday.com/media/images/author/EmilyHutto_188.jpg)

300x240.png)