How Are Diamonds Made? Natural vs Lab-Created Explained
Two Paths, One Diamond Not all diamonds come from the same place — but they all start the same way. Pure carbon, crystalized under immense pressure and heat. Whether it…
Nineteen years ago the Gemological Institute of America, (GIA), published the results of a human experiment in the observation of diamonds with various amounts of blue fluorescence. The article was entitled “A Contribution to Understanding the Effect of Blue Fluorescence on the Appearance of Diamonds”. An introductory editorial indicated that this study “should bring into question the trade’s lower “bid” prices for moderate to highly fluorescent diamonds in the better colors”.
GIA was addressing the negative publicity concerning blue fluorescent diamonds, which began during the diamond investment craze of the late 70’s, early 80’s. Since then blue fluorescence has been an obstacle to marketing, leading to discounting compared to non-fluorescent diamonds of the same color grade.
There are several reasons for the concern and distrust by consumers and the trade of these gemstones that glow blue when excited in the dark by ultra violet (UV) radiation, Figure 2. The reasons are mostly due to misinformation and misguided publicity except for one valid concern. That is the overgrading of color that according to members of the diamond trade is too often observed. Overgrading results from the use of UV-containing, fluorescent lighting in color grading.
In reaction to the GIA study’s conclusions Martin Rapaport commented in the April 1998 issue of the “Rapaport Diamond Report”: “Unfortunately, the probability of a lab overgrading a fluorescent stone is much greater than a non-fluorescent stone and a large percentage of high color mistakes turn out to be fluorescent.” “Obviously from the market perspective there appears to be a reasonable basis for price discrimination against fluorescence. The labs are going to have to be very serious about not overgrading the color of fluorescent stones even though these stones tend to appear whiter than they are”.
Here is a summary of the history and controversy over blue fluorescent diamonds and their color grading. This issue has been on a low boil since GIA published their study and experiment in late 1997. That was 19 years ago. However, the problem of misrepresentation and the misgrading of fluorescent diamonds goes way back, even before its documentation by Wade in 1916, and well before GIA’s founding.

Fast forward to today. Gemologists are advised to use unfiltered UV-containing fluorescent lighting that approximates northern daylight as the standard for color grading. This requirement for UV in the lighting is an abandonment of the grading principles adhered to by the laboratories and the diamond trade up to and through the 90’s. In addition, the variability of UV in fluorescent lighting is a cause of inconsistent grading of fluorescent diamonds.
Eric Bruton’s book, “Diamonds”, indicated that gemologists worldwide shared these views on illumination for diamond color grading. He said a “very important consideration is that any fluorescence in the stone must be suppressed”….”It is therefore important to grade stones in white light that is relatively free of ultra-violet”.
The problem continues today due to the almost universal use of fluorescent lighting in diamond color grading. The following excerpts are from the study article “The overgrading of blue-fluorescent diamonds: the problem, the proof and the solutions” from The Journal of Gemmology / 2010 that explain the problem and proof of the problem along with easy to implement solutions.
The light yellowish tints in a type 1a diamond, which comprise 98% of gem quality diamonds, combine with the various amounts of blue fluorescence, excited by UV and Visible Violet (VV), to give blue-fluorescent diamonds a whiter “perceived color” than is seen in lighting where fluorescence is not noticeably stimulated. Wade said “Some of these stones are inferior in beauty to pure white stones when viewed under a light which does not cause them to fluoresce.”[Wade, 1916]
Not known to the trade until recently is that blue fluorescence is stimulated not just by UV, but also by the narrow band of “visible-violet” (VV) wavelengths from 390nm up to 415.2nm (the center of the absorption band of the N3 aggregate). The energy in this band is too weak to cause grade whitening fluorescence at typical viewing distances from artificial illumination, where a diamond’s unimproved color is seen.
The following three pages contain a detailed discussion and documented proof of this issue that is then followed by solutions and conclusions.
Not long after Robert Shipley founded the GIA in 1931, he recruited academic members to a GIA advisory board to help advance the gemology movement in America. Chief among these members, especially in the field of diamond science and valuation was Frank Wade. Wade was a pioneer in America of “the first series of scientific articles (from 1915 to 1948) on diamonds and gems written especially for the jeweler” [Gilbertson, A., 2007].
Given his own studies and input from diamond experts and educators like Wade, it is no surprise to find Shipley concerned about fluorescence in the color grading of diamonds. He addressed this fluorescent diamond grading problem in Gems and Gemology, 1941. There he says: “One of the most important causes of the anomalies that so often trouble a diamond grader is the change of color shown by many fluorescent stones when viewed under different light conditions. Often a fluorescent diamond, which appears slightly yellowish under artificial light, appears distinctly bluish in daylight.”
The problem is how to correctly grade blue-fluorescing diamonds, when they often appear a higher, whiter color grade in daylight than their color seen indoors under typical artificial lighting. The diamond’s color unimproved by fluorescence is observed at normal wearing and viewing distances from most all forms of artificial illumination including fluorescent lighting. We note from Wade that more than 30 years before the founding of the GIA, the trade valued a diamond based upon what the trade called its “true color”, as seen indoors in artificial light, not its fluorescence improved color.
In daylight, additional factors, including time of day, geographic location, and whether or not the day was sunny or cloudy cause the color of a fluorescent diamond to change. With the perceived color of fluorescent diamonds varying with the illumination, what lighting should be used in laboratory color grading?
Historically, the standard lighting for color grading was the equivalent of “northern daylight” such as that through the North facing windows of the Israel Diamond Exchange pictured in Figure 3, but with the UV component removed to avoid stimulation of grade whitening fluorescence.

In the 50’s, the GIA switched to a new DiamondLite, Figure 4, that used the Verilux brand of fluorescent lighting, which they believed and taught gave “a north daylight balanced illumination—minus the UV.”
GIA’s winter 1997 G&G article on “The Effect of Blue Fluorescence on the Appearance of Diamonds” marked a change in GIA thinking and teaching. The study revealed a surprising amount of UV in the DiamondLite. This discovery was especially surprising to gemologists who were taught into the 90’s that the DiamondLite employed “fluorescent lamps designed to produce light fairly close to noon sunlight with insignificant amounts of ultraviolet” in order to grade diamond color unimproved by blue fluorescence.
Digital radiometer readings by GIA revealed a similar long wave UV content in each source of fluorescent lighting including the Verilux tubes in the standard DiamondLite. They also found “indirect daylight through our windows has about as much UV radiation as the fluorescent light sources”.

With the finding that “fluorescent lighting” and “daylight through a window” have a similar amount of UV radiation, it would be expected that blue-fluorescing diamonds would be perceived to be whiter in daylight through a window and in the DiamondLite than they would when viewed at typical distances from indoor, artificial lighting where there is negligible UV. Rather than grading a diamond’s “true color” unenhanced by fluorescence, those using the DiamondLite with Verilux tubes were grading a diamond’s fluorescence-improved color.
The amount of UV exciting fluorescence in the diamond being graded varies with the fluorescent tube’s manufacturer, with the tube’s size, wattage, age, and most significantly with the distance the diamond is held from the tube during grading. Despite there being a large UV component as measured at two to seven inches in fluorescent diamond grading light, at typical viewing distances from similar overhead fluorescent lights the UV is reduced to less than a micro watt. This variability produces varying amounts of blue fluorescence causing inconsistent color grading.
A closer look at the Isreal Diamond Exchange in Figures 3 and 5 reveals hundreds of fluorescent desk lamps being used in diamond grading. Solutions are needed to correct the problem of overgrading due to the almost universal use of fluorescent lights to color-grade diamonds at grading laboratories and diamond exchanges throughout the world.
In his 2009 Gems and Gemology letter to the editor, Thomas Tashey recounts: “I was shocked when I made the initial discovery, by placing a clear, UV filter, plastic film between the Verilux lamps in the DiamondLite and the diamonds to be graded, that stones with very strong blue fluorescence could change to a lower color by three or four letter grades.” He spoke of a 0.89ct marquise brilliant with Very Strong Blue fluorescence: “In the DiamondLite [Verilux lamps, without UV filter] this stone was graded table down as a high “D”. … When viewed table down, with the UV filter between the lamps and the diamond, the color grade of the diamond shifted to that of a low “H”.” Tashey also found that diamonds with “medium” to “strong” blue fluorescence generally shifted one to two grades when the filter was used. (The Professional Gemologist, 2000)
This example in a Very-Strong-Blue fluorescent marquise diamond, of a four and a half grade color improvement to high D in the DiamondLite over its unenhanced color grade of low H, may be met with disbelief by professionals in the trade, most all of whom grade in some form of UV-containing fluorescent illumination, and so have not witnessed this large a shift in color. However, the 25-diamond data base in Cowing, 2010. “The overgrading of blue-fluorescent diamonds” contains a 0.63ct marquise diamond with the same close to five grade improvement in the DiamondLite over its color unimproved by fluorescence.
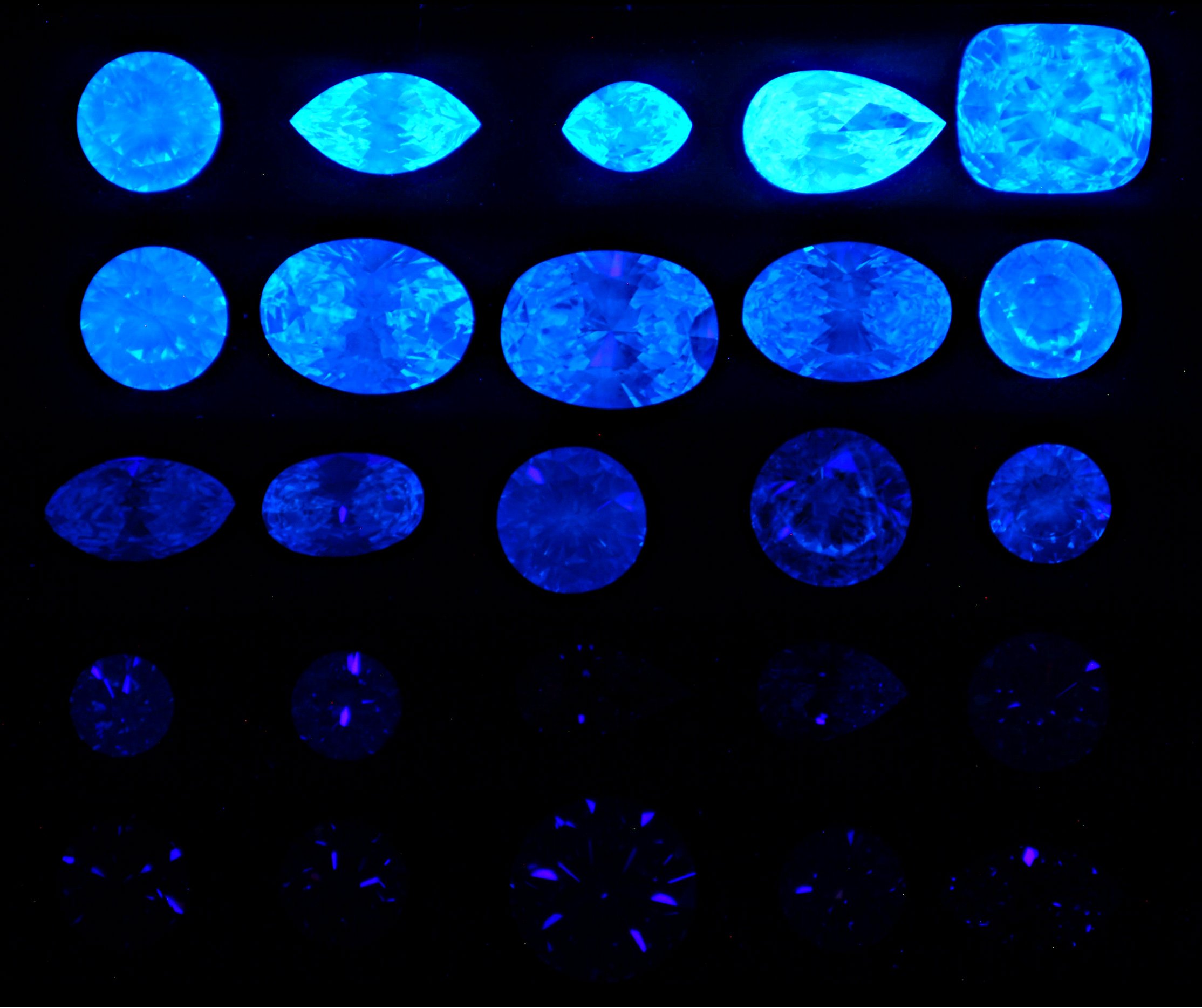
Documentation of the overgrading of blue-fluorescent diamonds was unintentionally contained in photography, Figure 6a, in the winter 1997 Gems and Gemology article on “A Contribution to Understanding the Effect of Blue Fluorescence on the Appearance of Diamonds”.
The four color sets of diamonds used in these observation studies were carefully chosen from more than 1000 diamonds, so they would be similar to one another in all respects except their fluorescence. Their colors were graded in the daylight equivalent fluorescent illumination of the DiamondLite. This illumination, with its unfiltered daylight fluorescent tubes, was the standard from the 60’s to 2000 for grading diamond color at the GIA Gem Trade Labs.
Color photographs were published with the article of each of the four, six stone sets whose colors were E, G, I and K.

The four face-up photographs were taken in the same incandescent illumination (pers. comm. photographer, Erica Van Pelt), which (by its nature and intensity) was relatively UV-free. Consequently, these photographs present the opportunity to observe the relative colors among the diamonds in each set unimproved by blue fluorescence.
If there were no overgrading, diamonds within each set should look the same color. But they do not. Look, for example, at the face up view of the 6 stone I color set in the top row of Figure 6a, and consider that all these stones are graded “I” color.

The I color set’s diamonds have fluorescent strength, from left to right, of: 1. Medium, 2. Very Strong, 3. Faint, 4.Strong, 5. None, and 6. Strong. If you study those six stones in the face up photograph, stones 2, 4 and 6 appear to have substantially more color than the other three in spite of having been graded identically as I ‘s.
It is no coincidence that these are the three with the strongest blue fluorescence. Revealed in this relatively UV-free lighting is the darker color unenhanced by blue fluorescence of the strongly fluorescing members of this I color set. The considerable difference in color of these six identically graded I color diamonds is perfectly correlated with their fluorescent strength. The lower true color is revealed, because this photograph was taken in lighting lacking sufficient UV and VV to stimulate blue fluorescence. Here is photographic documentation that the strong and very strong fluorescing I colors in the GIA study have been overgraded in the UV-containing and fluorescence-stimulating illumination of the DiamondLite.
Photography and printing only crudely render these variations of color, and the other sets are less consistent in showing this effect, but the highest fluorescing member of each of the E, G, and K color sets appears to be the most tinted of its respective color set. From a color grader’s perspective these obvious color differences are documentation of significant overgrading.
Take the right-most two diamonds, one non-fluorescent and the other strong blue. They are carefully oriented in the photograph with centered culets, tables perpendicular to the camera lens, and are placed adjacent to each other in the same illumination, yet the Strong Blue diamond is clearly more tinted.
The difference in the right most two diamonds is easier to see in Figure 6b from the combination of the same upper-right halves of those two diamonds into one image that matches the sizes of each half while maintaining the color differences between the diamonds as they appear in Figure 6a.

While the difference is apparent, the magnitude of overgrading relative to the “true color” unenhanced by fluorescence cannot be accurately quantified from this photograph. That quantification was accomplished by analysis of the grading of the 25 diamond data base in Cowing’s study. The grading was done by not only the author, but also by both GIA GTL, and AGSL.
When diamonds are viewed in social situations at night or indoors away from daylight, it is usually at distances of a few feet from artificial illumination. In these circumstances the “true color” is seen, as the UV and visible violet are too weak to stimulate grade-whitening fluorescence. This is in contrast to most color grading environments where the diamond is typically 2 to 7 in. from fluorescent lighting with significant UV and VV components.
Today gemologists are advised to use unfiltered UV-containing fluorescent lighting that approximates northern daylight as the standard for color grading. This requirement for UV in the lighting is an abandonment of the diamond trade’s grading principles adhered to by GIA into the 90’s. In addition, the variability of UV in fluorescent lighting is a cause of inconsistent grading of fluorescent diamonds.
The desired grade for a blue-fluorescent diamond should be re-established as that color seen in typical artificial lighting where fluorescence is not noticeably stimulated.
The measurements and data in the 2010 study confirm that the UV and VV energy in fluorescent and other indoor artificial illumination decreases rapidly with distance from the source. The diamond’s color unenhanced by blue fluorescence is seen at usual viewing distances from artificial lighting where UV is typically less than one microwatt.
Reduction in UV with distance affords a partial solution to the overgrading of blue-fluorescent diamonds. Partial because Lab grading is done from about 2 to 7 in. from fluorescent tube lighting containing significant, grade whitening UV and VV. Increases in grading distance over that range can help, but do not solve the problem of overgrading in diamonds having fluorescent strengths of ‘Medium Blue’, ‘Strong Blue’ and especially not ‘Very Strong Blue’.
Reducing the UV by increasing the grading distance was employed by GIA with their change at the labs in 2000 from grading in the DiamondLite to requiring grading in the tray of the DiamondDock. See Figure 7. That change was from grading at 2 to 3 inches from the DiamondLite tubes to the DiamondDock with its grading tray seven inches from the tubes. The result was a change in the amount of UV excitation from upwards of 150µW/cm2 in the DiamondLite to the vicinity of 30 µW/cm2 at the grading tray in the DiamondDock; a five times reduction. The study found that this amount of UV reduction takes the typical amount of overgrading in Very Strong Blues from as much as four and a half grades down to two grades. With this change in the standard grading light, the potential for overgrading was significantly reduced but not eliminated.

A complete solution to overgrading , which adequately eliminates UV in the grading illumination, and at the same time does not materially affect grading the diamonds D-Z tints of yellow is the use of polycarbonate plastic, such as Lexan or Makrolon. As first demonstrated by Tashey in 2009 polycarbonate is an effective and inexpensive filter to block UV below 385 nm removing its grade whitening effect on blue fluorescent diamonds.
To reduce fluorescence stimulated by visible violet to that of typical artificial lighting, an equally effective and inexpensive solution is the use of flat white plastic diffusers, which attenuate violet and all visible wavelengths equally. Below 400 fc or about 4000 lux, the reduced amount of visible violet did not excite noticeable fluorescence. Such white diffusers have the additional feature of reducing spectral reflections and glare. They were employed on GIA microscope lights for this purpose and to filter UV.
Another solution demonstrated in this study is the use of white LED technology. In this investigation, a Dazor LED desk lamp not only provided inherently UV-free grading light, but was dimmable without change in its daylight color temperature down to 2000–4000 lux (200–400 fc), to avoid noticable stimulation of fluorescence from VV.
Nearly two decades have passed, and the problem remains due to the almost universal use of fluorescent lighting in diamond color grading. The result is continuing distrust of blue fluorescent diamonds with the consequent discounting required to sell them.
From Wade’s time to this day gemologists and the trade refer to the diamond’s color unimproved by fluorescence as its “true color.” It is the color commonly seen in a diamond at typical viewing distances from artificial illumination at night or indoors out of daylight. There the light at the diamond contains insufficient UV (less than one microwatt) to stimulate grade whitening fluorescence.
Restoration of grading for the diamond’s true color can be accomplished by the use of polycarbonate plastic such as Lexan. Polycarbonate is an effective and inexpensive filter that blocks the UV in fluorescent lighting, removing its grade whitening effect on blue fluorescent diamonds. Another solution is the use of white LED technology. LED lighting provides inherently UV-free grading light avoiding noticable stimulation of fluorescence.
Either solution is consistent with the trade’s historical desire that diamonds be examined for their unenhanced “true body color” in lighting largely free of UV.
A return to the practice of grading a diamond’s true color rather than its fluorescence enhanced color would benefit the diamond industry in several ways.
First it would remove the distrust and stigma attached to fluorescent diamonds.
Second, the rarer blue-fluorescent diamonds that hold their high-white color in the absence of fluorescence stimulating UV and VV would be recognized for their superior beauty and rarity to diamonds that drop in color.
Thirdly, blue-fluorescent diamonds could be shown to whiten from their graded color, and sometimes appear blue-white in natural daylight. Promoting this advantage in comparison with non-fluorescent diamonds of similar grade would return the marketing advantage to blue fluorescent diamonds that they once enjoyed.
By grading in lighting that does not stimulate fluorescence, fairness and consistency can be achieved, restoring trust in and rekindling desire for this outstanding gemstone.
Thank you to the following individuals for their contributions, discussions, editing, and suggestions: The AGA Task Force on Lighting Standards, especially Dazor Manufacturing’s Stan Hogrebe and Ann Simpson; gem and jewelry author, Antoinette Matlins; Thomas Hainschwang; Professor Alan Collins; current and former GIA, Ron Geurtz, Al Gilbertson, Karen Hurwit, Phil Yantzer, Thomas Tashey, Gary Roskin; current AGSL Peter Yantzer, Jason Quick, Katie Overton and Jennifer Tobiasson; David Federman; Michael Shields, and gemologist-author Richard Cartier.
Cowing, M.D., 1998. “Issues of Color Grading Blue
Fluorescent Diamonds”, NAJA Quarterly
Cowing, M.D., 2010. “The overgrading of bluefluorescent
diamonds: the problem, the proof and the
solutions”, The Journal of Gemmology, vol. 32/Nos 1-4,
38-51
Gemological Institute of America, 1969. The GIA
Diamond Course, Assignment #35, 3-6
Gilbertson, A.M., 2007. American Cut: The First
100 Years. Gemological Institute of America, Carlsbad,
CA. 214 pp
King, J.M., Geurts, R.H., Gilbertson, A., and
Shigley, J.E., 2008. Color grading “D-to-Z” diamonds
at the GIA Laboratory. Gems & Gemology, 44(4),
296–321
Moses, T., Reinitz, I.M., Johnson, M.L., King, J.M.,
and Shigley, J.E, 1997. The effect of blue fluorescence
on the appearance of diamonds. Gems & Gemology,
33(4), 244–59
Moses, T., 2001. Response to The Professional
Gemologist article regarding fluorescence in
diamonds. The Professional Gemologist, 3(2), 1–2
Rapaport, M., 1998. “Blue White”, Rapaport
Diamond Report.
Shipley, R.M., and Liddicoat, R.T. Jr., 1941. A
solution to diamond color grading problems. Gems &
Gemology, 3(11),162–80
Tashey, T., 2000. The effect of fluorescence on the
color grading and appearance of white and offwhite
diamonds. The Professional Gemologist, 3(1), 5–7
Tashey, T., 2009. Letters. More on D-Z diamond
color grading. Gems & Gemology, 45(2) 51–2
Wade, F.B., 1916.
Diamonds. G.P. Putnam’s Sons, New York and London,
150 pp
Gemological Institute of America, 1969. The GIA Diamond Course, Assignment #35, 3-6
A large percentage of diamonds fluoresce, usually blue; and the fluorescence, if sufficiently intense, will alter the color of such a stone when observed under a light source emitting ultraviolet rays. Since this occurs under daylight examination, the most desirable conditions are to be encountered under a balanced artificial light with a minimum of ultraviolet content.
The DiamondLite consists of a metal grading box containing a filament-type bulb and a daylight filter that produce the equivalent of north light but without ultraviolet radiation.
For those jewelers who do not have access to a DiamondLite, a reasonably good substitute can be made by adapting a simple desk-lamp fixture containing cool-white fluorescent tubes. The disadvantage of this kind of illumination is that the fluorescent tubes emit a significant percentage of ultraviolet radiation. Although this does not affect the grading of nonfluorescent stones, it causes fluorescent diamonds to be graded higher than is actually warranted, due to the neutralizing, or masking, effect of the fluroescent color on the true body color.
Gemological Institute of America, 1979. The GIA Diamond Course, Assignment #19, 7-9
One of the primary requirements for effective diamond color grading is standard lighting. Although daylight, at its best, provides excellent illumination for distinguishing faint nuances of color, it is not a satisfactory standard light source for diamond color grading for reasons (among which) is that the presence of ultraviolet in sunlight will make some stones that exhibit blue fluroescence appear higher in color. Fluorescent diamonds should be graded at their color in artificial light devoid of ultraviolet radiation, rather than at their daylight appearance.
Fluorescent tubes, which produce a balanced light, and are practically devoid of ultraviolet waves, are being manufactured. These tubes are used in the color grading instrument developed by GIA under the trade name DiamondLite.
Gemological Institute of America, 1994. The GIA Diamond Course, Assignment #2, 10-11
The traditional standard for grading color was north daylight. This is no longer the case; daylight is excellent for distinguishing faint nuances of color, but, for a number of reasons, it is not a dependable light source for diamond color grading: 1. It varies at different times of the year – or even from day to day. 2. It also varies from place to place, and is affected by variables such as atmospheric conditions, smog, dust, and clouds. 3. The ultraviolet in sunlight causes some stones to fluoresce, which changes their apparent color.
The light from most ordinary fluorescent lamps contains too much ultaviolet for accurate color grading. “Daylight” fluorescent lamps are designed to produce light fairly close to noon sunlight with insignificant amounts of ultraviolet. They are used in the DiamondLite, a color grading instrument developed and marketed by GIA GEM Instruments, and in some overhead-light attachments for microscopes.
King, Geurts, Gilbertson, and Shigley, 2008. Color grading “D-to-Z” diamonds at the GIA Laboratory. Gems & Gemology, 44(4), 296–321
Diamonds are graded on a white tray placed on the DiamondDock shelf, which enforces a seven inch grading distance from the tubes. These twin seventeen inch Verilux fluorescent tubes and the seven inch grading distance define the current light standard for diamond grading at GIA GTL. From the G&G Winter 2008 article on colour grading at GIA GTL, p305, the basic technical specifications for the lighting used for D-Z color grading are:
GIA notes regarding the UV (in the GIA standardized fluorescent light source): “we have learned that for some fluorescent diamonds the distance between the lamps and the grading tray can influence the final color grade. For consistency, we use a distance of 8-10 in. between the lamps and the diamond. Bringing a fluorescent diamond closer to the lamps may result in a stronger fluorescent impact.”
With the change in standard lighting at the Laboratory to the two, 17in. 20-watt fluorescent lamps in the DiamondDock, GIA has gone from requiring that diamonds be graded in light with a minimum of UV to requiring that “the lamp should emit long-wave UV, which is an important characteristic of daylight.“ This is the lighting that GIA said “causes fluorescent diamonds to be graded higher than is actually warranted, due to the neutralizing, or masking, effect of the fluroescent color on the true body color.”
Although a grading distance of 8-10 inches is specified, the shelf of the DiamondDock enforces a 7 inch grading distance from lamps to grading tray. Because UV and VV vary greatly with distance from the lamps the exact distance is important to establish, because it effectively defines the chosen “standard” amount of UV excitation. GIA researcher Ronald Geurtz (pers. com.) notes an important point about this current lighting standard. The allowed range of light intensity of 2000-4500lux at the surface of the grading tray means the “standard” amount of UV and VV also varies over this same 2.5 times range.
Such an allowed large variation of UV and VV defeats standardization of the amount of UV. The variability of UV in fluorescent lighting remains a cause of inconsistency in the grading of fluorescent diamonds.