- Joined
- Jun 8, 2008
- Messages
- 56,762
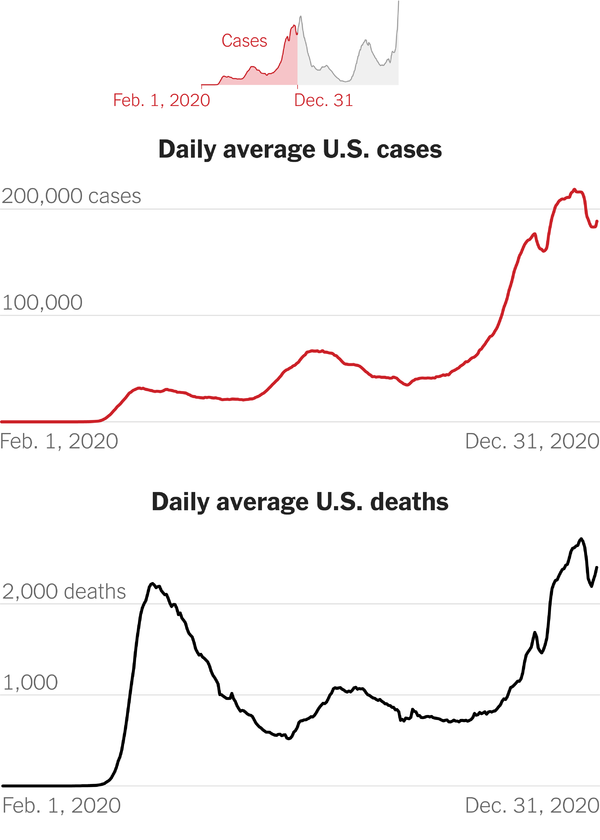
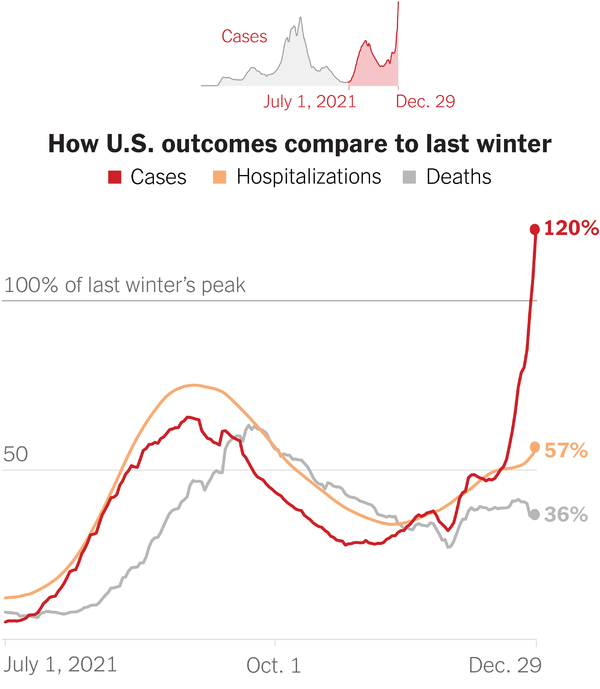
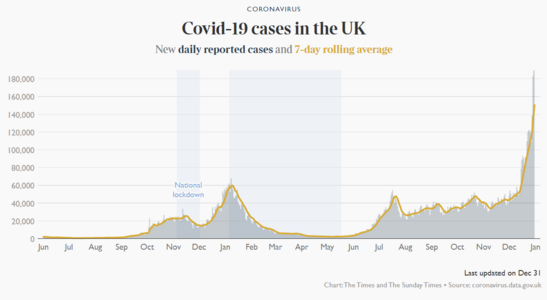
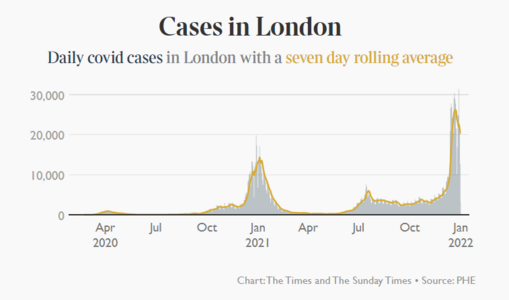
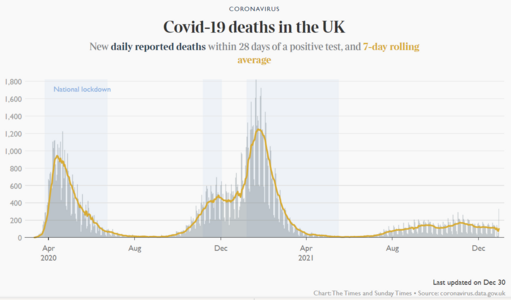
Wow two years into the pandemic.
Here we are.
Definitely much further along and better off despite a surge at the moment.
"
The ongoing COVID-19 pandemic was the biggest medical news or event of 2021, according to an overwhelming 84% of 987 of our readers who answered a recent Medscape Medical News poll. Perhaps no surprise there.
Coming in distant second, at 26%, was the new law requiring that patients be granted electronic access to clinical notes. The controversial FDA approval of aducanumab (Aduhelm, Biogen/Eisai) to treat Alzheimer's disease was next, cited by almost 16% when asked what they would remember most about 2021.
Coming in at 10% or less were the permanent end to the Step 2 Clinical Skills test, the JAMA deputy editor resignation over controversial comments, and an "other" option that allowed for write-in responses.
It should be noted respondents could choose up to three answers to this and other questions in this survey, except for questions about profession and specialty.
FDA clearance of a 5-minute test for early dementia was selected by 22%, followed by almost 16% citing approval in October 2021 of abemaciclib(Verzenio, Lilly) "described as the first advance for early breast cancer in 20 years."
The resignation of JAMA editors over a podcast on race rounded out the list of exciting medical news or events ― coming in fourth at 11%. A total 5% of readers chose "other" and were asked to specify what news or events excited them in 2021.
Medscape also asked readers what medical news or events frustrated them in 2021. A majority, 81%, chose COVID-19 vaccine hesitancy or refusal. Almost one third, 31%, chose the effect of climate change on health worldwide.
Some of the most memorable news or events of 2021 were also selected as frustrating by readers. For example, 22% were frustrated by the law requiring that patients be granted electronic access to clinical notes, followed by 19% who referred to the aducanumab approval in June. Furthermore, about 12% selected the JAMA resignations.
The US Preventive Services Task Force ruling out aspirin in people over age 60 for primary prevention of cardiovascular disease shocked 36% of respondents.
Coming in third and fourth on the survey were the two JAMA editors resigning after a podcast on race, chosen by 19%, and the demise of the Step 2 Clinical Skills test, selected by 18%.
Interestingly, almost 96% of respondents were physicians. Less than 1% were residents, physician assistants, or nurses. Respondents also represented a wide range of specialties. From a list of 29 possible specialties, including "other," family medicine, internal medicine, and psychiatry were the most common.
For more on the year that was 2021, see the Medscape Year in Medicine 2021: News That Made a Difference slideshow. Read Medscape's full Year in Medicine report.
Wondering what stood out most to our readers in 2020? Here is a story about the results of a similar survey 1 year ago.
"
Here we are.
Definitely much further along and better off despite a surge at the moment.
"
COVID-19, Sure, but What Else Will We Remember 2021 For?
Damian McNamara, MAThe ongoing COVID-19 pandemic was the biggest medical news or event of 2021, according to an overwhelming 84% of 987 of our readers who answered a recent Medscape Medical News poll. Perhaps no surprise there.
Coming in distant second, at 26%, was the new law requiring that patients be granted electronic access to clinical notes. The controversial FDA approval of aducanumab (Aduhelm, Biogen/Eisai) to treat Alzheimer's disease was next, cited by almost 16% when asked what they would remember most about 2021.
Coming in at 10% or less were the permanent end to the Step 2 Clinical Skills test, the JAMA deputy editor resignation over controversial comments, and an "other" option that allowed for write-in responses.
It should be noted respondents could choose up to three answers to this and other questions in this survey, except for questions about profession and specialty.
Exciting News in 2021
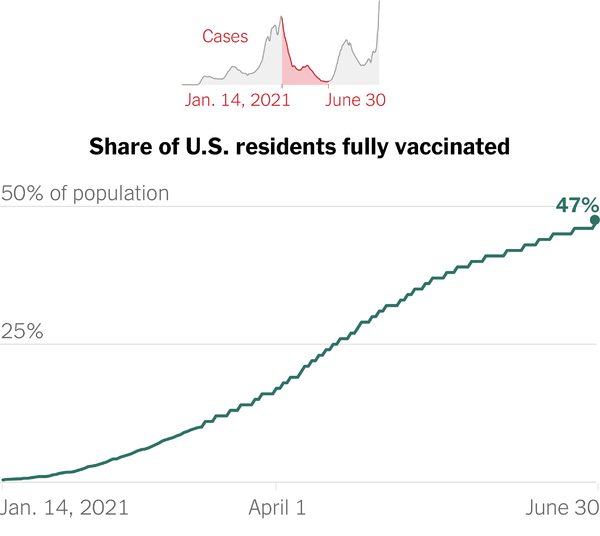
Widespread availability of COVID-19 vaccines was the number one response ― chosen by 85% ― when asked what medical news or events excited them in 2021.FDA clearance of a 5-minute test for early dementia was selected by 22%, followed by almost 16% citing approval in October 2021 of abemaciclib(Verzenio, Lilly) "described as the first advance for early breast cancer in 20 years."
The resignation of JAMA editors over a podcast on race rounded out the list of exciting medical news or events ― coming in fourth at 11%. A total 5% of readers chose "other" and were asked to specify what news or events excited them in 2021.
A Frustrating Year?
Medscape also asked readers what medical news or events frustrated them in 2021. A majority, 81%, chose COVID-19 vaccine hesitancy or refusal. Almost one third, 31%, chose the effect of climate change on health worldwide.
Some of the most memorable news or events of 2021 were also selected as frustrating by readers. For example, 22% were frustrated by the law requiring that patients be granted electronic access to clinical notes, followed by 19% who referred to the aducanumab approval in June. Furthermore, about 12% selected the JAMA resignations.
A Shocking Survey Question
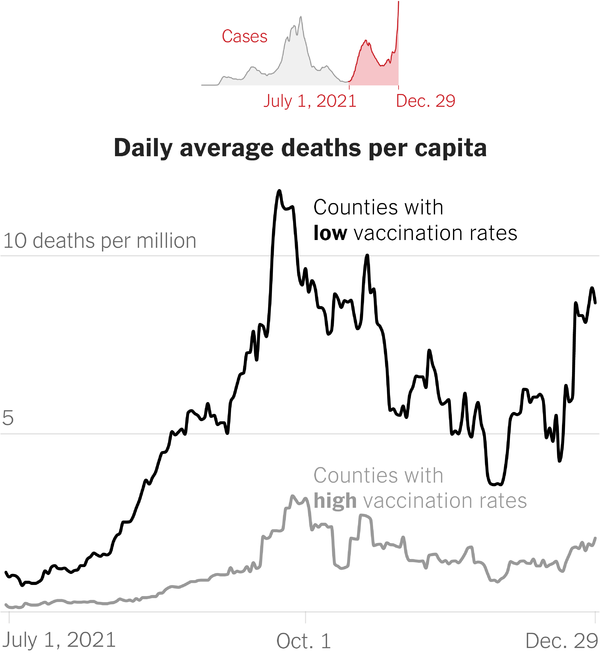
Asked what medical news or event from 2021 shocked readers, COVID-19 vaccine hesitancy or refusal was the most common answer, at 69%.The US Preventive Services Task Force ruling out aspirin in people over age 60 for primary prevention of cardiovascular disease shocked 36% of respondents.
Coming in third and fourth on the survey were the two JAMA editors resigning after a podcast on race, chosen by 19%, and the demise of the Step 2 Clinical Skills test, selected by 18%.
Interestingly, almost 96% of respondents were physicians. Less than 1% were residents, physician assistants, or nurses. Respondents also represented a wide range of specialties. From a list of 29 possible specialties, including "other," family medicine, internal medicine, and psychiatry were the most common.
For more on the year that was 2021, see the Medscape Year in Medicine 2021: News That Made a Difference slideshow. Read Medscape's full Year in Medicine report.
Wondering what stood out most to our readers in 2020? Here is a story about the results of a similar survey 1 year ago.
"











300x240.png)