MMtwo
Ideal_Rock
- Joined
- Sep 20, 2009
- Messages
- 5,207
Australia is wild! We went from locking down playgrounds to being reasonable about Omicron. I suspect they think public goodwill is going to expire soon if they lock us up again.

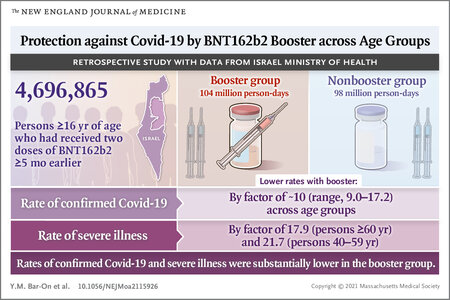
Omicron ‘five times less severe’ than Delta
NSW Premier Dominic Perrottet says early signs indicate Omicron is “five times less severe” than Delta.www.news.com.au
That said I REALLY REALLY REALLY trust Dr Chant and I do everything she tells me (seriously I ate dodgy supermarket bread for that lady because she said not to go into cramped bakeries) so if she's going to hang her hat on Omicron being milder, I believe her.
I saw that! Talk about a complete and utter about face in policy.
I have also been following along with the more encouraging reports out of countries that have data on hospitalization and severity and am so hopeful that this is a pivotal point for everyone.















300x240.png)