- Joined
- Jun 8, 2008
- Messages
- 56,270
This is for my friend Nikki @Daisys and Diamonds
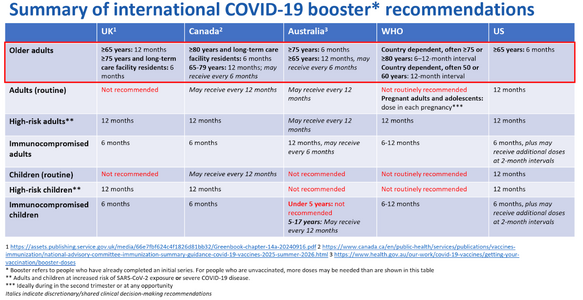
Not much to update but here’s what I have

 www.medpagetoday.com
www.medpagetoday.com

 www.medpagetoday.com
www.medpagetoday.com

 www.medscape.com
www.medscape.com

 kffhealthnews.org
kffhealthnews.org
Let me know if you can’t open these. I’m on my phone and it’s too hard to copy and paste all of it but when I get home I can if there’s anything you need me to copy and paste
Feel better Nicky
Not much to update but here’s what I have

Retrospective Study Links COVID in Kids With Post-Acute Kidney Outcomes
Findings underscore importance of long-term monitoring for some children and adolescents

Army Enlists 3 Soldiers Ousted for Refusing COVID Vaccine, Corrects Other Numbers
Soldiers also expect to be given back pay

Experts: New Bat Coronavirus Being Watched
Expert advocates continued vigilance related to recent announcement of newly discovered coronavirus.

RFK Jr.’s Purge of FOIA Staff at FDA Spares People Working on Covid Vaccine Lawsuits - KFF Health News
A purge of FDA staff spared some people tasked with responding to a judge’s orders to disclose government records on covid vaccines, according to agency employees. The FOIA litigation was brought by Aaron Siri, an ally of Health and Human Services Secretary Robert F. Kennedy Jr.'s who represents...
Let me know if you can’t open these. I’m on my phone and it’s too hard to copy and paste all of it but when I get home I can if there’s anything you need me to copy and paste
Feel better Nicky






300x240.png)