- Joined
- Mar 11, 2013
- Messages
- 1,637
I am not sure entirely, but leaning towards getting my 2nd booster, just not sure when. Is the government covering the cost still? If not, I am going on Medicare in June (already 65 just on my DH's insurance and he is retiring end of May.) So do not know if it makes a difference if I get it now with my regular insurance or better if Medicare pays?
DD said she is not getting a 2nd booster as she is still recovering from myocarditis from the 1st booster. She won't have her 2nd echocardiogram until May to see if everything has resolved, though she says the rapid heartbeats are not happening as often. The cardiologist said he is not sure what she should do and many of his colleagues are confused as well when it comes to booster shots.
I believe the government is covering the cost still. In various cities and counties they have free COVID vaccine clinics, some every weekend for example. Maybe see if your county has info on free clinics in your area. (I follow my county health department on Twitter to see what’s available and discussions of COVID topics.)


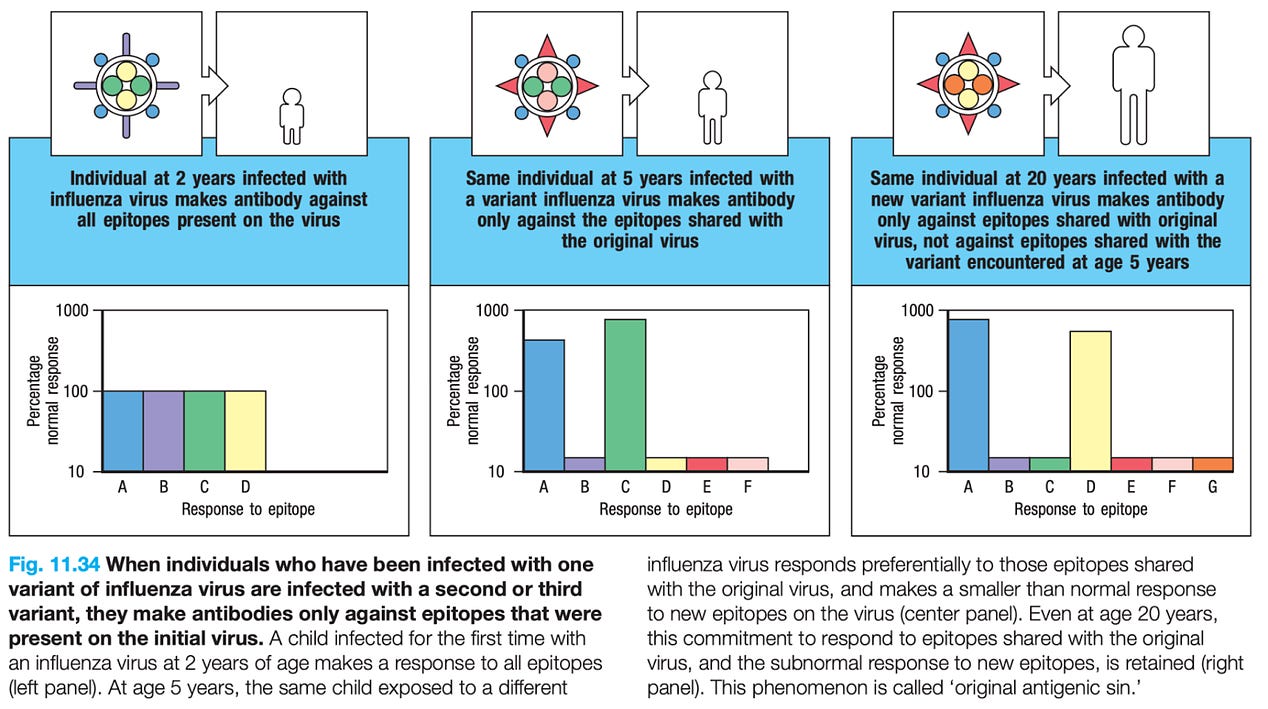








300x240.png)