- Joined
- Jun 8, 2008
- Messages
- 54,265
"

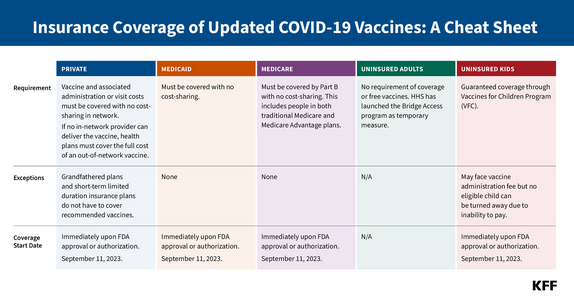
The FDA has authorized and approved updated mRNA COVID-19 vaccinesopens in a new tab or window for use in adults and children ages 6 months and up, the agency announced on Monday.
Doing away with any vestige of the original vaccines -- the bivalent shots targeted both BA.4/5 and the wild-type strain -- the new vaccines from Pfizer-BioNTech and Moderna are monovalent products that solely target the XBB.1.5 Omicron subvariant, following recommendations from an FDA advisory panelopens in a new tab or window that convened in June.
The updated vaccines -- approved for people 12 years and up and under emergency authorization for kids 6 months to 11 years of age -- can be used for primary series or booster vaccinations, and with this new action, the previous bivalent shots are no longer authorized for use in the U.S.
While XBB.1.5 now only makes up 3% of U.S. cases, according to CDC's Nowcast trackeropens in a new tab or window, the shots are still expected to offer broad protection against circulating strains. EG.5, dubbed Erisopens in a new tab or window, is currently responsible for the largest share of cases in the nation (22%), followed by FL.1.5.1 (15%) and then mostly a slew of XBB strains.
A particular concern of late has been the BA.2.86 or Pirola strainopens in a new tab or window, which has yet to register on CDC's tracker. Vaccine makers Modernaopens in a new tab or window and Pfizeropens in a new tab or window have assured, however, that their XBB.1.5-adapted products can effectively neutralize the highly mutated variant. The extent of neutralization appears "to be of a similar magnitude to the extent of neutralization observed with prior versions of the vaccines against corresponding prior variants against which they had been developed to provide protection," the FDA stated.
Under the FDA's action, people 5 years and older can receive a single dose of the updated mRNA vaccines at least 2 months since their last dose of any COVID vaccine.
For kids 6 months to 4 years, those who have been previously vaccinated will be eligible for one or two doses of the new products, depending on how many previous doses they have received. Those currently unvaccinated can receive three doses of Pfizer's vaccine or two doses of Moderna's vaccine.
Under the new approval, primary series vaccinations in people 12 years and older for Moderna and Pfizer's products call for a single dose (down from two doses).
"Vaccination remains critical to public health and continued protection against serious consequences of COVID-19, including hospitalization and death," Peter Marks, MD, PhD, director of the FDA's Center for Biologics Evaluation and Research, said in a statement. "The public can be assured that these updated vaccines have met the agency's rigorous scientific standards for safety, effectiveness, and manufacturing quality. We very much encourage those who are eligible to consider getting vaccinated."
It's widely expected that CDC will recommend the updated boosters for older adults and other vulnerable groups -- such as immunocompromised individuals and those with established medical conditions placing them at high risk for severe outcomes from COVID-19 -- though it's less clear how forceful recommendations will be for younger, healthy populations.
Uptake of the bivalent vaccine, authorized last summer, was limited, with only 17% of the total population opting for a shotopens in a new tab or window to date. That rate reached 43% for adults 65 and over.
Full recommendations for use of the new monovalent vaccines will be made at a meeting of CDC's Advisory Committee on Immunization Practices (ACIP) scheduled for Tuesday, September 12opens in a new tab or window.
"
FDA OKs Updated COVID Shots
— New single-strain vaccines will exclusively target the XBB.1.5 Omicron subvariant
by Ian Ingram, Managing Editor, MedPage Today September 11, 2023
The FDA has authorized and approved updated mRNA COVID-19 vaccinesopens in a new tab or window for use in adults and children ages 6 months and up, the agency announced on Monday.
Doing away with any vestige of the original vaccines -- the bivalent shots targeted both BA.4/5 and the wild-type strain -- the new vaccines from Pfizer-BioNTech and Moderna are monovalent products that solely target the XBB.1.5 Omicron subvariant, following recommendations from an FDA advisory panelopens in a new tab or window that convened in June.
The updated vaccines -- approved for people 12 years and up and under emergency authorization for kids 6 months to 11 years of age -- can be used for primary series or booster vaccinations, and with this new action, the previous bivalent shots are no longer authorized for use in the U.S.
While XBB.1.5 now only makes up 3% of U.S. cases, according to CDC's Nowcast trackeropens in a new tab or window, the shots are still expected to offer broad protection against circulating strains. EG.5, dubbed Erisopens in a new tab or window, is currently responsible for the largest share of cases in the nation (22%), followed by FL.1.5.1 (15%) and then mostly a slew of XBB strains.
A particular concern of late has been the BA.2.86 or Pirola strainopens in a new tab or window, which has yet to register on CDC's tracker. Vaccine makers Modernaopens in a new tab or window and Pfizeropens in a new tab or window have assured, however, that their XBB.1.5-adapted products can effectively neutralize the highly mutated variant. The extent of neutralization appears "to be of a similar magnitude to the extent of neutralization observed with prior versions of the vaccines against corresponding prior variants against which they had been developed to provide protection," the FDA stated.
Under the FDA's action, people 5 years and older can receive a single dose of the updated mRNA vaccines at least 2 months since their last dose of any COVID vaccine.
For kids 6 months to 4 years, those who have been previously vaccinated will be eligible for one or two doses of the new products, depending on how many previous doses they have received. Those currently unvaccinated can receive three doses of Pfizer's vaccine or two doses of Moderna's vaccine.
Under the new approval, primary series vaccinations in people 12 years and older for Moderna and Pfizer's products call for a single dose (down from two doses).
"Vaccination remains critical to public health and continued protection against serious consequences of COVID-19, including hospitalization and death," Peter Marks, MD, PhD, director of the FDA's Center for Biologics Evaluation and Research, said in a statement. "The public can be assured that these updated vaccines have met the agency's rigorous scientific standards for safety, effectiveness, and manufacturing quality. We very much encourage those who are eligible to consider getting vaccinated."
It's widely expected that CDC will recommend the updated boosters for older adults and other vulnerable groups -- such as immunocompromised individuals and those with established medical conditions placing them at high risk for severe outcomes from COVID-19 -- though it's less clear how forceful recommendations will be for younger, healthy populations.
Uptake of the bivalent vaccine, authorized last summer, was limited, with only 17% of the total population opting for a shotopens in a new tab or window to date. That rate reached 43% for adults 65 and over.
Full recommendations for use of the new monovalent vaccines will be made at a meeting of CDC's Advisory Committee on Immunization Practices (ACIP) scheduled for Tuesday, September 12opens in a new tab or window.
"





















300x240.png)