Daisys and Diamonds
Super_Ideal_Rock
- Joined
- Apr 30, 2019
- Messages
- 23,323
so a big brave fireman can run into a burning building but is scared of a tiny needle ???
Stuff
www.stuff.co.nz
Only something like 60% of the FDNY and not much more of the NYPD are vaccinated. It blows my mind.

| ||
|
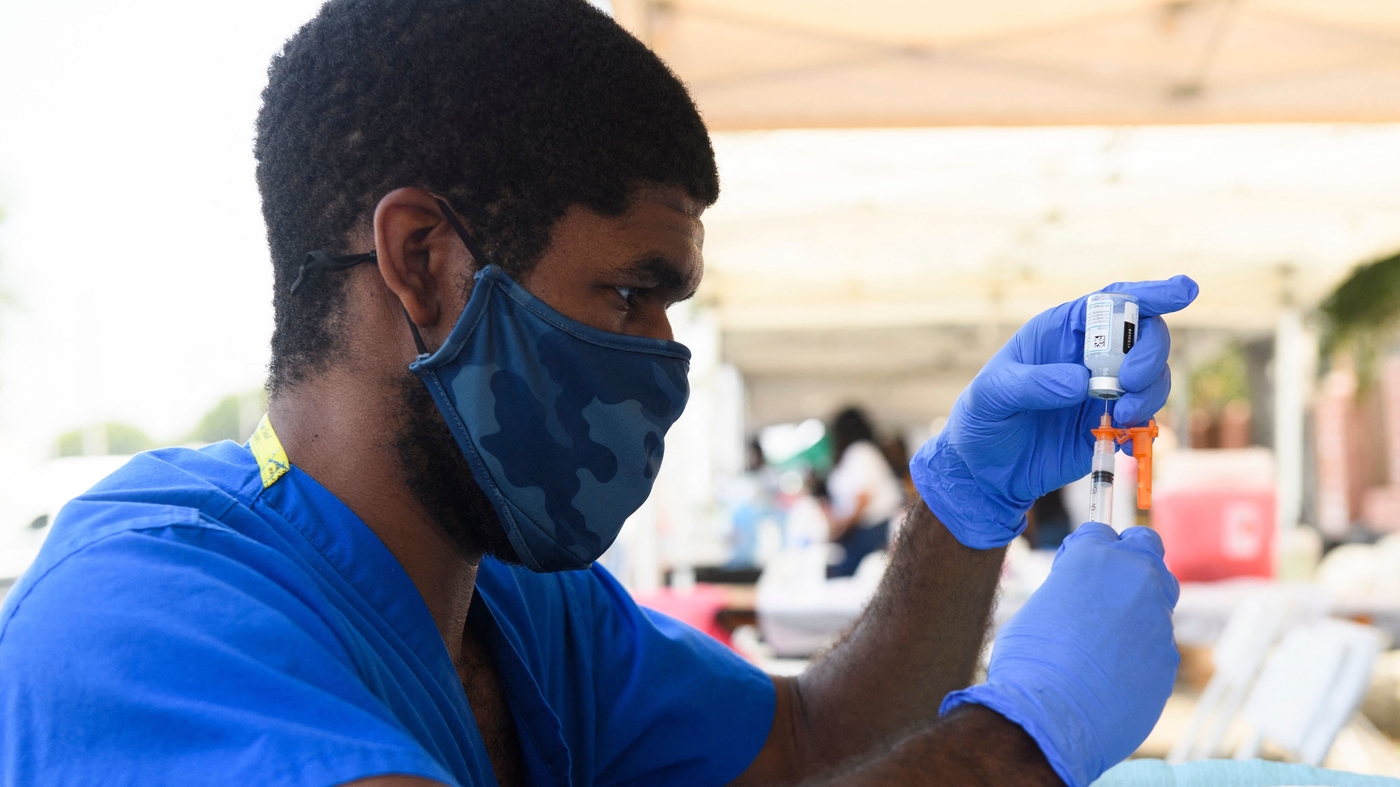
FDA has authorized Moderna and J&J booster, says it's ok to mix vaccines

The FDA authorizes Moderna and J&J COVID vaccine boosters
The Food and Drug Administration also gave an OK to boosters that differ from the vaccine originally used to immunize people against COVID-19. A mix-and-match approach could ease the booster rollout.www.npr.org
My FIL has COVID (vaccinated and symptomatic). He has been pretty careful but works in sanitation so is public facing. So far he says it feels similar to the flu. We're not too worried since he has been vaccinated but we're obviously hoping he recovers quickly and without needing medical intervention.
Just read this! Beat me to it!


| |||||
| |||||
| |||||
|
that is kewl@Karl_K , I called our local Osco today about getting my parents the booster. The pharmacist said they were taking walk-ins but said it was getting busy so scheduling an appointment would probably be best. He said appointments had to be scheduled online. They had all 3 vaccines available.

@dk168 I'm so sorry.
I know you were looking forward to seeing them.
Hopefully soon..better postpone now and you can see them safely later.
But I know it must be super disappointing. (((Hugs))).
Thanks missy.
I know I often say visiting my mum is duty and not a holiday, however, for once I am disappointed not being able to go.
Nevermind.
DK
And at the end of that statement, it is always followed up with "they told me they wished they got it and now its too late". My heart breaks every time. And there is nothing anyone can do
