- Joined
- Jun 8, 2008
- Messages
- 54,248
Good morning. Here's the November thread. 2022 edition. Hoping next year we don't need these threads anymore.
Here's the latest plus some miscellaneous issues
"
Anticipating a winter surge, the Biden administration extended the COVID-19 public health emergency through Jan. 11, 2023. (ABC News)
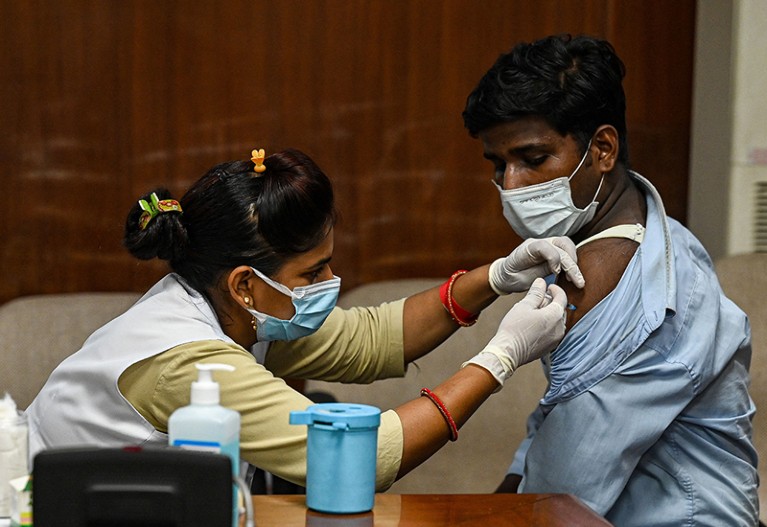
Another 3.3 million Americans received their booster jab over the past week, bringing the total number of boosted to 14.8 million, according to CDC data. (Reuters)
The Supreme Court let a ruling stand which allows the Transportation Security Administration to mandate mask-wearing on planes, trains and other forms of transportation. (The Hill)
CDC Director Rochelle Walensky, MD, MPH, developed COVID rebound after being treated with Paxlovid, the agency announced.
The FDA said that clinicians providing abortion pills to patients before they are pregnant -- a prescribing method known as advance provision -- are acting without the agency's authorization, and that they could be putting patients at risk. (Politico)
A judge in North Dakota stopped the state's abortion ban from going into effect, saying it's likely the law will face constitutional challenges once it's implemented. (AP)
Missouri's health department is investigating whether a hospital violated federal law by denying a woman an abortion in a medical emergency. (ABC News)
Ob/gyn residency programs in states that restrict abortion face a difficult choice when it comes to educating their trainees on abortion: risk prosecution or losing their accreditation. (New York Times)
Pfizer said its respiratory syncytial virus (RSV) vaccine for pregnant women was 81.8% effective in preventing severe infection in infants, stating it will submit an application for approval to the FDA by the end of this year.
Scientists are exploring therapies that target human proteins for the treatment of COVID-19. (Washington Post)
Shanghai Disneyland closed indefinitely yesterday due to the COVID pandemic. (NPR)
A record number of cholera outbreaks has been reported across the globe, forcing health agencies to ration their limited supply of vaccines. (New York Times)
The FDA warned of a shortage of tracheostomy tubes, urging providers to reuse tubes or use appropriate alternatives.
Abiomed announced that the FDA granted pre-market approval to its Impella RP Flex with SmartAssist, an implanted device to treat acute heart failure for up to 2 weeks.
The CDC issued a Health Alert Network advisory emphasizing the importance of cleaning and monitoring dental waterlines, following multiple nontuberculous Mycobacteria (NTM) infections in children who were exposed to water with high levels of bacteria at pediatric dental clinics.
Voters in Arkansas, Maryland, Missouri, North Dakota, and South Dakota -- four of which are among the most conservative states in the nation -- will decide whether or not to legalize recreational marijuana in the upcoming election. (NPR)
A man accused of intentionally setting fire to a Tennessee Planned Parenthood clinic died in August, officials said. (AP)
Experts explain why the latest wellness trend -- parasite cleanses -- is "modern snake oil." (Washington Post)
An Iowa egg farm home to 1.1 million chickens has been infected with bird flu. (ABC News)
"
Here's the latest plus some miscellaneous issues
"
Anticipating a winter surge, the Biden administration extended the COVID-19 public health emergency through Jan. 11, 2023. (ABC News)
Another 3.3 million Americans received their booster jab over the past week, bringing the total number of boosted to 14.8 million, according to CDC data. (Reuters)
The Supreme Court let a ruling stand which allows the Transportation Security Administration to mandate mask-wearing on planes, trains and other forms of transportation. (The Hill)
CDC Director Rochelle Walensky, MD, MPH, developed COVID rebound after being treated with Paxlovid, the agency announced.
The FDA said that clinicians providing abortion pills to patients before they are pregnant -- a prescribing method known as advance provision -- are acting without the agency's authorization, and that they could be putting patients at risk. (Politico)
A judge in North Dakota stopped the state's abortion ban from going into effect, saying it's likely the law will face constitutional challenges once it's implemented. (AP)
Missouri's health department is investigating whether a hospital violated federal law by denying a woman an abortion in a medical emergency. (ABC News)
Ob/gyn residency programs in states that restrict abortion face a difficult choice when it comes to educating their trainees on abortion: risk prosecution or losing their accreditation. (New York Times)
Pfizer said its respiratory syncytial virus (RSV) vaccine for pregnant women was 81.8% effective in preventing severe infection in infants, stating it will submit an application for approval to the FDA by the end of this year.
Scientists are exploring therapies that target human proteins for the treatment of COVID-19. (Washington Post)
Shanghai Disneyland closed indefinitely yesterday due to the COVID pandemic. (NPR)
A record number of cholera outbreaks has been reported across the globe, forcing health agencies to ration their limited supply of vaccines. (New York Times)
The FDA warned of a shortage of tracheostomy tubes, urging providers to reuse tubes or use appropriate alternatives.
Abiomed announced that the FDA granted pre-market approval to its Impella RP Flex with SmartAssist, an implanted device to treat acute heart failure for up to 2 weeks.
The CDC issued a Health Alert Network advisory emphasizing the importance of cleaning and monitoring dental waterlines, following multiple nontuberculous Mycobacteria (NTM) infections in children who were exposed to water with high levels of bacteria at pediatric dental clinics.
Voters in Arkansas, Maryland, Missouri, North Dakota, and South Dakota -- four of which are among the most conservative states in the nation -- will decide whether or not to legalize recreational marijuana in the upcoming election. (NPR)
A man accused of intentionally setting fire to a Tennessee Planned Parenthood clinic died in August, officials said. (AP)
Experts explain why the latest wellness trend -- parasite cleanses -- is "modern snake oil." (Washington Post)
An Iowa egg farm home to 1.1 million chickens has been infected with bird flu. (ABC News)
"













![author['full_name'] author['full_name']](https://clf1.medpagetoday.com/media/images/author/shannonFirth_188.jpg)


300x240.png)