Undercover999
Rough_Rock
- Joined
- Nov 15, 2011
- Messages
- 62
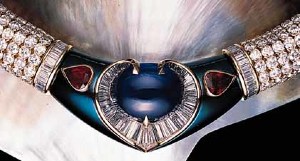
Calling all pics of blue gold - alloy, intermetallic, oxide, or inteference coating, lets see em!
I've done a bunch of googling but I'm hoping you pricescopers have some stuff I haven't already found/seen. Thanks!
I've done a bunch of googling but I'm hoping you pricescopers have some stuff I haven't already found/seen. Thanks!



























300x240.png)